

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50362900 (CHEMBL1945284) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute for Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A light chain using SNAP-25 (187 to 203) as substrate by HPLC analysis | ACS Med Chem Lett 2: 396-401 (2011) Article DOI: 10.1021/ml200028z BindingDB Entry DOI: 10.7270/Q2JD4X80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50362899 (CHEMBL1945167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute for Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A light chain using SNAP-25 (187 to 203) as substrate by HPLC analysis | ACS Med Chem Lett 2: 396-401 (2011) Article DOI: 10.1021/ml200028z BindingDB Entry DOI: 10.7270/Q2JD4X80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50362898 (CHEMBL1945166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
U.S. Army Medical Research Institute for Infectious Diseases Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A light chain using SNAP-25 (187 to 203) as substrate by HPLC analysis | ACS Med Chem Lett 2: 396-401 (2011) Article DOI: 10.1021/ml200028z BindingDB Entry DOI: 10.7270/Q2JD4X80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50308030 (5-chloro-7-((4-ethoxyphenyl)(pyridin-3-ylamino)met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A protease activity using Ac-SNKTRIDEANQRATKML-NH2 as substrate after 5 mins by RP-HPLC analysis | ACS Med Chem Lett 3: 387-391 (2012) Article DOI: 10.1021/ml200312s BindingDB Entry DOI: 10.7270/Q2KK9CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50388866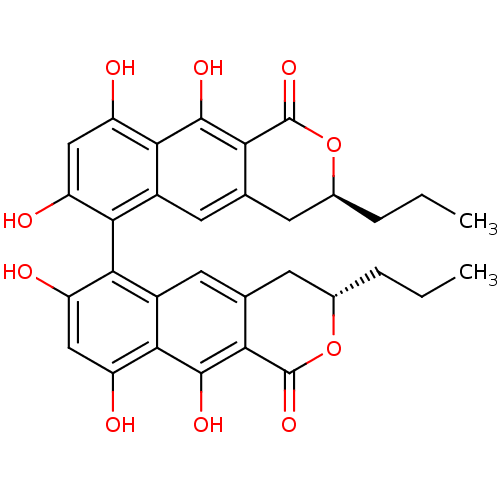 (CHEMBL2062928) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A protease activity using Ac-SNKTRIDEANQRATKML-NH2 as substrate after 5 mins by RP-HPLC analysis | ACS Med Chem Lett 3: 387-391 (2012) Article DOI: 10.1021/ml200312s BindingDB Entry DOI: 10.7270/Q2KK9CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50388866 (CHEMBL2062928) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A protease activity using Ac-SNKTRIDEANQRATKML-NH2 as substrate after 5 mins by RP-HPLC analysis | ACS Med Chem Lett 3: 387-391 (2012) Article DOI: 10.1021/ml200312s BindingDB Entry DOI: 10.7270/Q2KK9CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50388865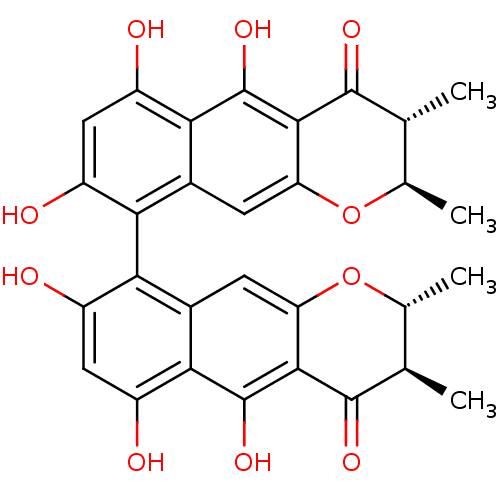 (CHAETOCHROMIN A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A protease activity using Ac-SNKTRIDEANQRATKML-NH2 as substrate after 5 mins by RP-HPLC analysis | ACS Med Chem Lett 3: 387-391 (2012) Article DOI: 10.1021/ml200312s BindingDB Entry DOI: 10.7270/Q2KK9CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50388867 (SECALONIC ACID A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A protease activity using Ac-SNKTRIDEANQRATKML-NH2 as substrate after 5 mins by RP-HPLC analysis | ACS Med Chem Lett 3: 387-391 (2012) Article DOI: 10.1021/ml200312s BindingDB Entry DOI: 10.7270/Q2KK9CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50269745 (CHEMBL463175 | Cephalochromin | cid_160115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A protease activity using Ac-SNKTRIDEANQRATKML-NH2 as substrate after 5 mins by RP-HPLC analysis | ACS Med Chem Lett 3: 387-391 (2012) Article DOI: 10.1021/ml200312s BindingDB Entry DOI: 10.7270/Q2KK9CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50388868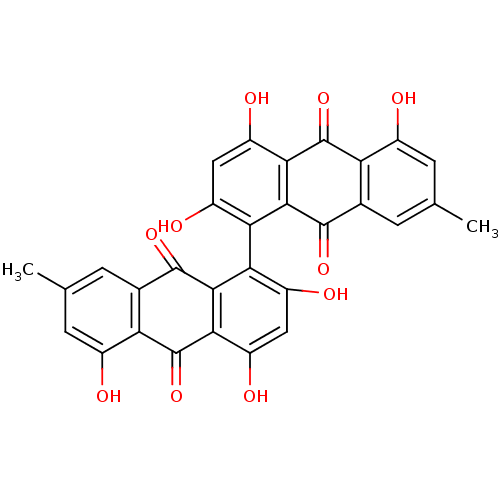 (CHEMBL472851) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum Hall BoNT/A protease activity using Ac-SNKTRIDEANQRATKML-NH2 as substrate after 5 mins by RP-HPLC analysis | ACS Med Chem Lett 3: 387-391 (2012) Article DOI: 10.1021/ml200312s BindingDB Entry DOI: 10.7270/Q2KK9CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||