

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521345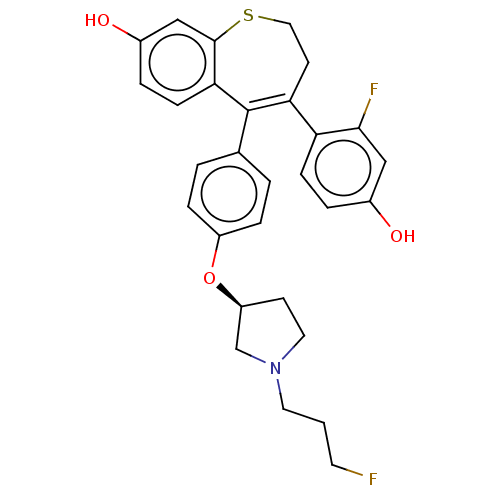 (4-(2-fluoro-4- hydroxy- phenyl)-5-[4- [(3S)-1-(3- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521334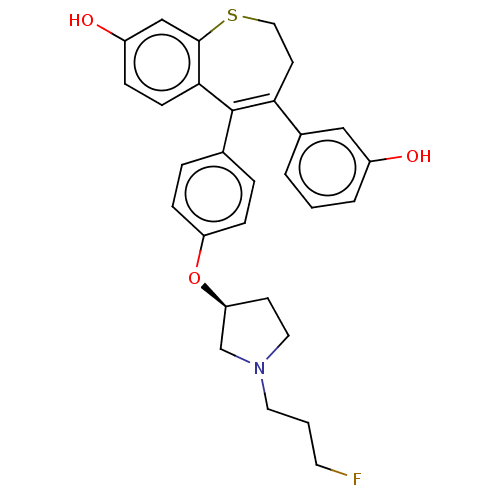 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521421 (6-(2,4- dichlorophenyl)- 1-fluoro-5-[6- [(3S)-1-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521296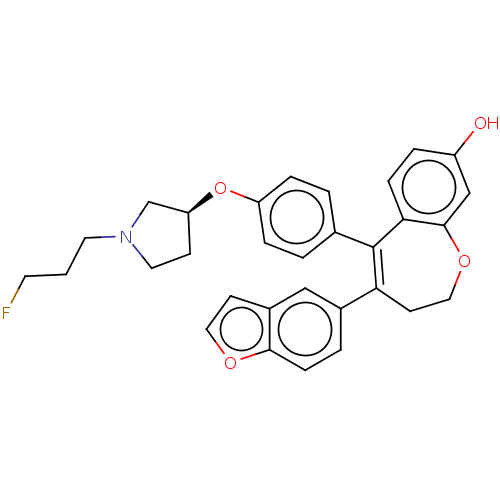 (4-(benzofuran- 5-yl)-5-[4-[(3S)- 1-(3- fluoropropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521416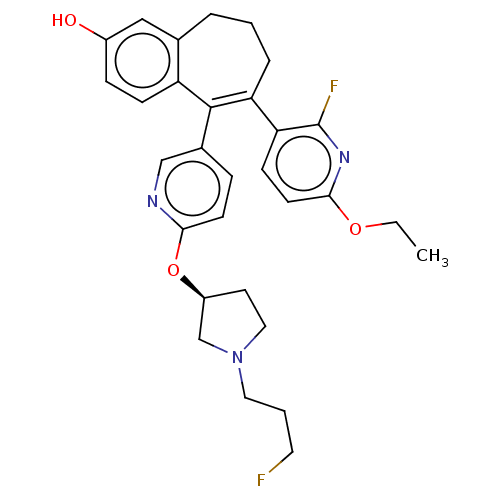 (6-(6-ethoxy-2- fluoro-3- pyridyl)-5-[6- [(3S)-1-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521360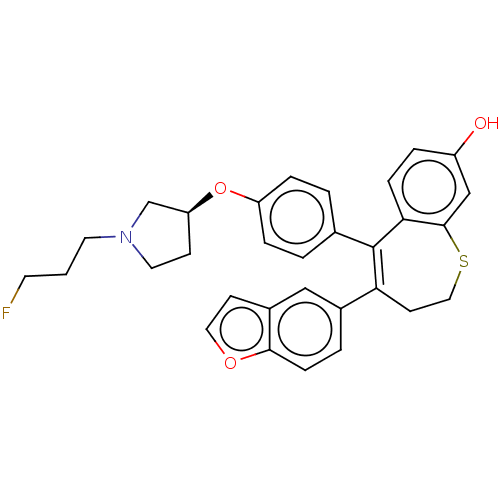 (4-(benzofuran- 5-yl)-5-[4-[(3S)- 1-(3- fluoropropy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521255 (3-(6-ethoxy-2- fluoro-3- pyridyl)-4-[6- [(3S)-1-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521384 (4-(6-ethoxy-2- fluoro-3- pyridyl)-5-[6- [(3S)-1-(3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521413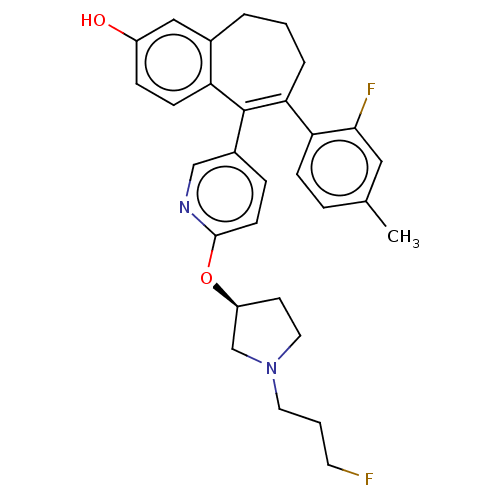 (6-(2-fluoro-4- methyl-phenyl)- 5-[6-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521262 (6-(6-ethoxy-2- fluoro-3- pyridyl)-5-[4- [[(3S)-1-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521270 (4-(2-fluoro-4- hydroxy- phenyl)-5-[4- [(3S)-1-(3- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521383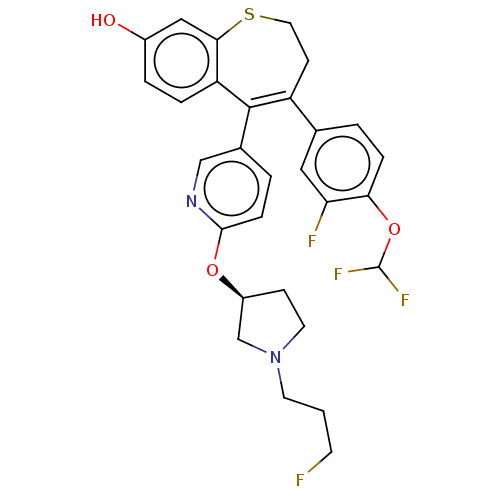 (4-[4- (difluoromethoxy)- 3-fluoro- phenyl]-5-[6- [...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521295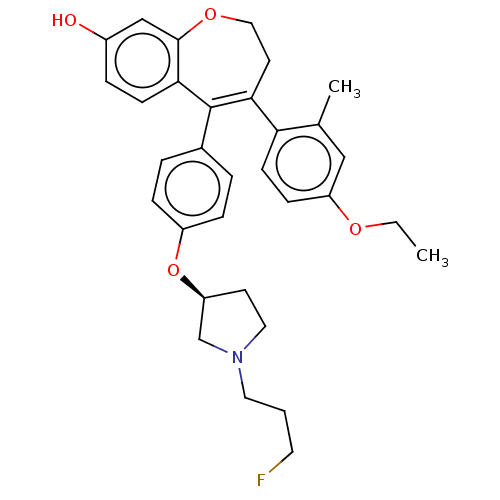 (4-(4-ethoxy-2- methyl-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521374 (4-(2,2- dimethylindolin- 5-yl)-5-[4-[(3S)- 1-(3- f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521267 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521192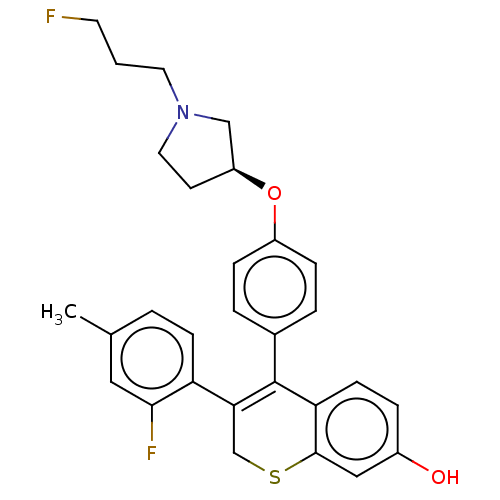 (3-(2-fluoro-4- methyl-phenyl)- 4-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521354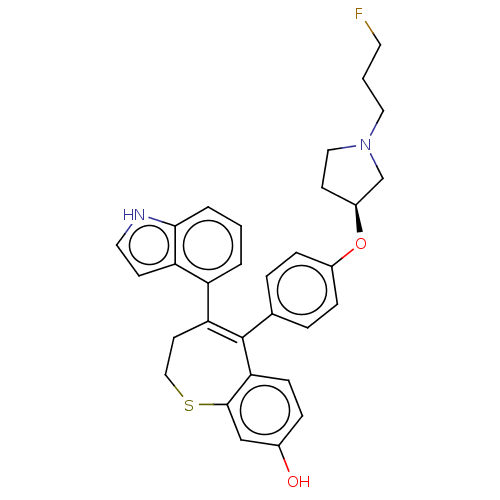 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521418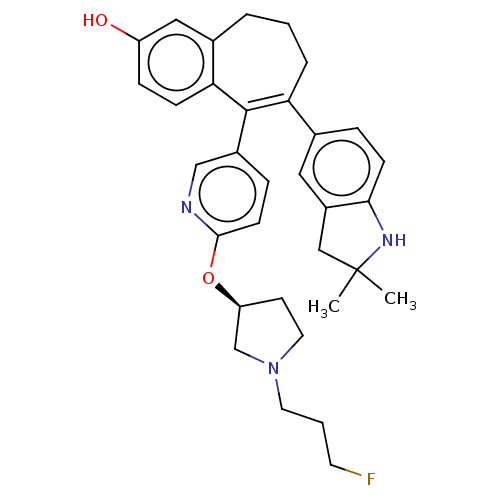 (6-(2,2- dimethylindolin- 5-yl)-5-[6-[(3S)- 1-(3- f...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521182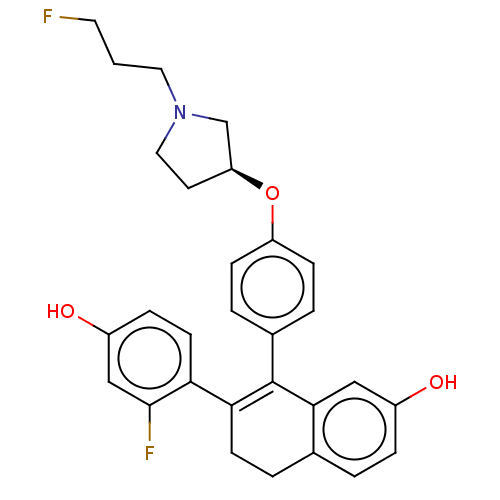 (7-(2-fluoro-4- hydroxy- phenyl)-8-[4- [(3S)-1-(3- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521341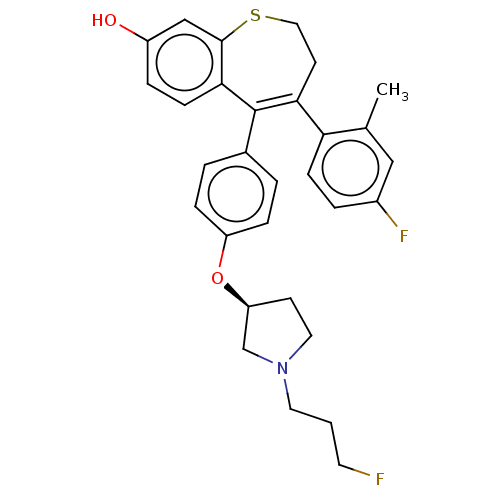 (4-(4-fluoro-2- methyl-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521336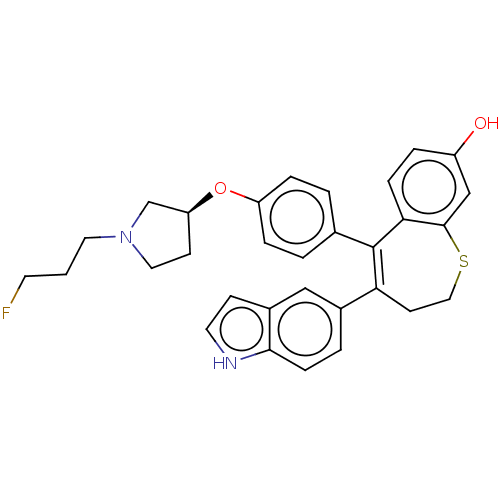 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521198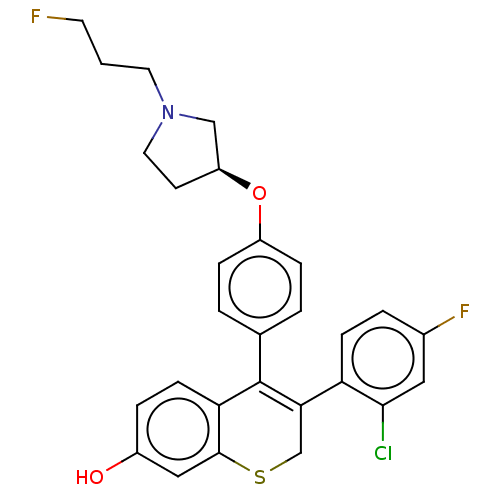 (3-(2-chloro-4- fluoro-phenyl)- 4-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521415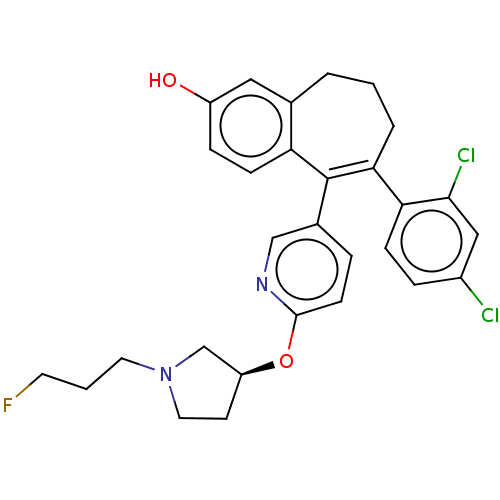 (6-(2,4- dichlorophenyl)- 5-[6-[(3S)-1-(3- fluoropr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521382 (4-(2-chloro-4- methyl-phenyl)- 5-[6-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521344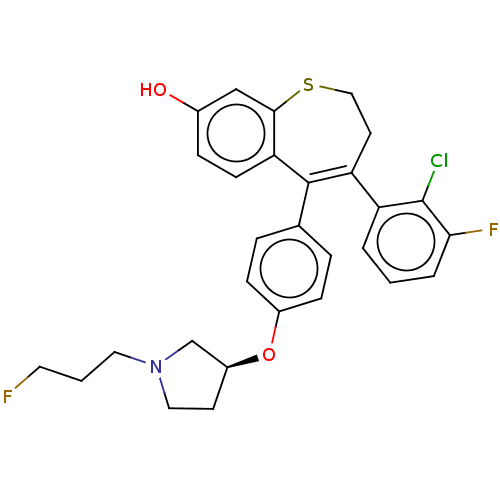 (4-(2-chloro-3- fluoro-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521428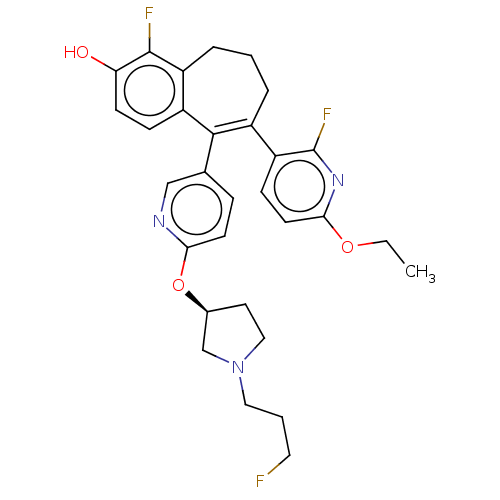 (6-(6-ethoxy-2- fluoro-3- pyridyl)-1- fluoro-5-[6- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521340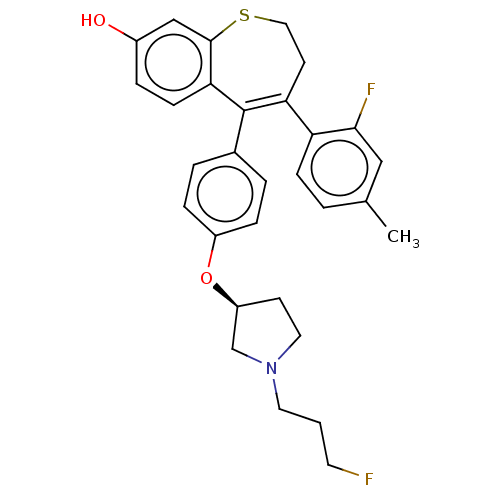 (4-(2-fluoro-4- methyl-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521335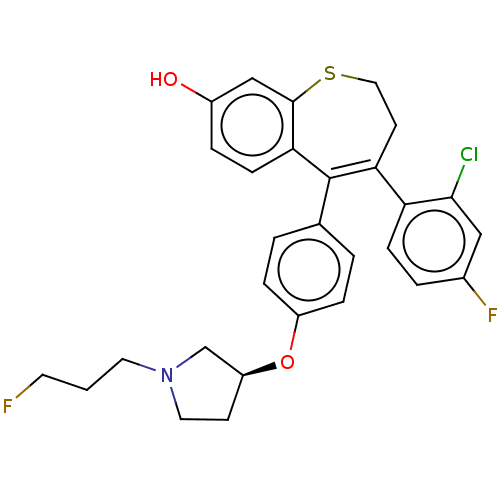 (4-(2-chloro-4- fluoro-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521378 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521381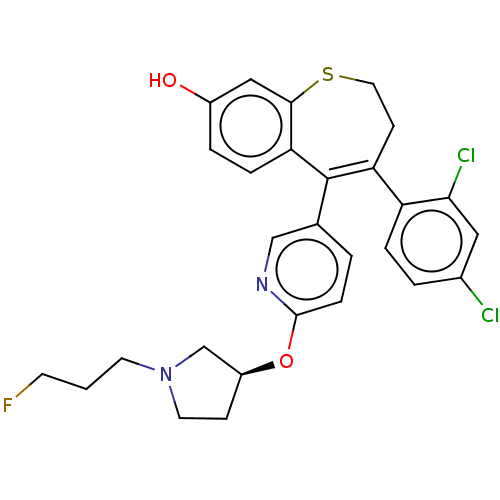 (4-(2,4- dichlorophenyl)- 5-[(6-[(3S)-1-(3- fluorop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521195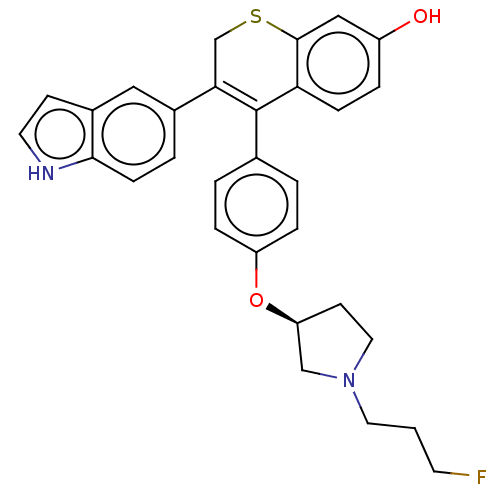 (4-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521297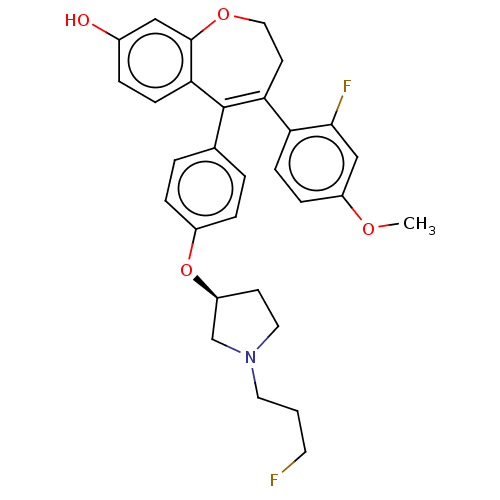 (4-(2-fluoro-4- methoxy- phenyl)-5-[4- [(3S)-1-(3- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521373 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521425 (6-[4- (difluoromethoxy)- 3-fluoro- phenyl]-1- fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521221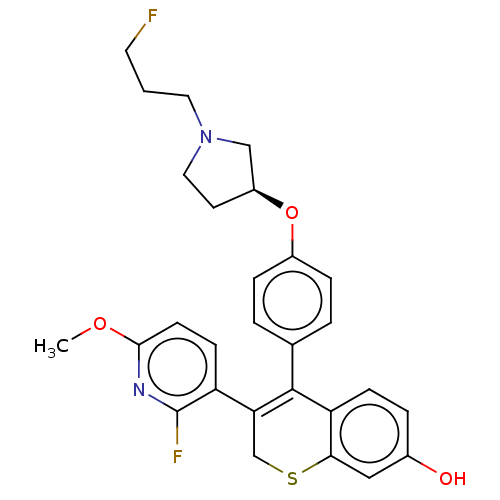 (3-(2-fluoro-6- methoxy-3- pyridyl)-4-[4- [(3S)-1-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521193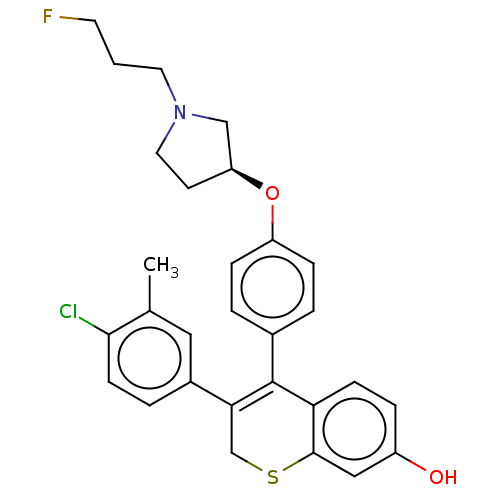 (3-(4-chloro-3- methyl-phenyl)- 4-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521377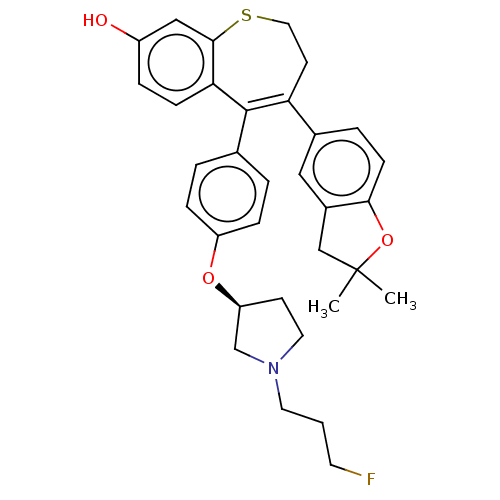 (4-(2,2-dimethyl- 3H-benzofuran- 5-yl)-5-[4-[(3S)- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521380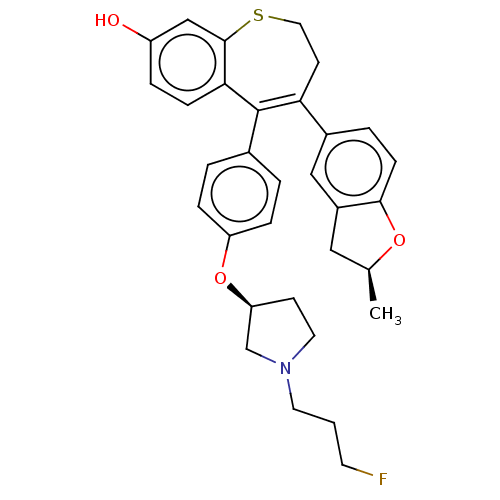 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521261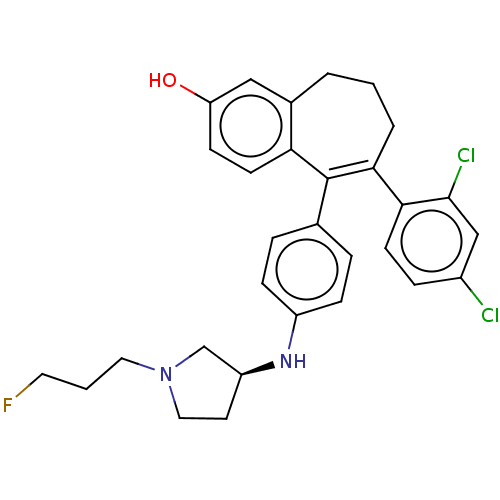 (6-(2,4- dichlorophenyl)- 5-[4-[[(3S)-1- (3- fluoro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521254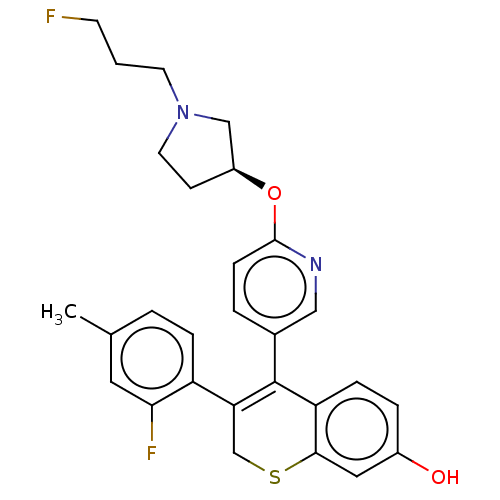 (3-(2-fluoro-4- methyl-phenyl)- 4-[6-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521194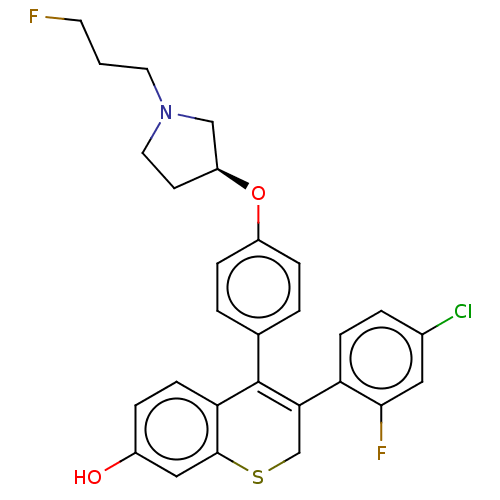 (3-(4-chloro-2- fluoro-phenyl)- 4-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521343 (4-(4-chloro-2- fluoro-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521356 (4-(4-fluoro-2- methyl-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521282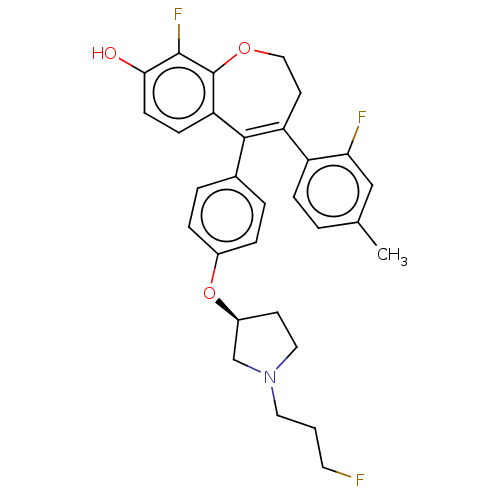 (9-fluoro-4-(2- fluoro-4-methyl- phenyl)-5-[4- [(3S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521417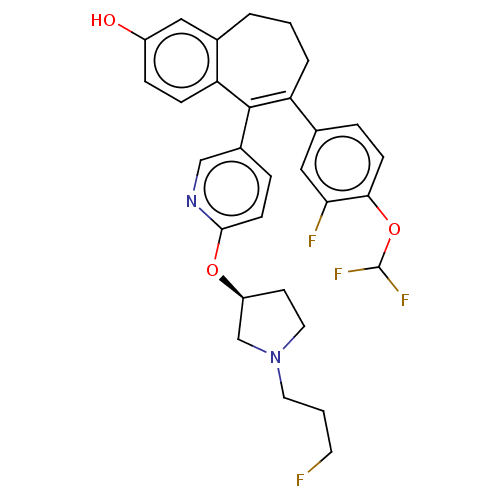 (6-[4- (difluoromethoxy)- 3-fluoro- phenyl]-5-[6- [...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521362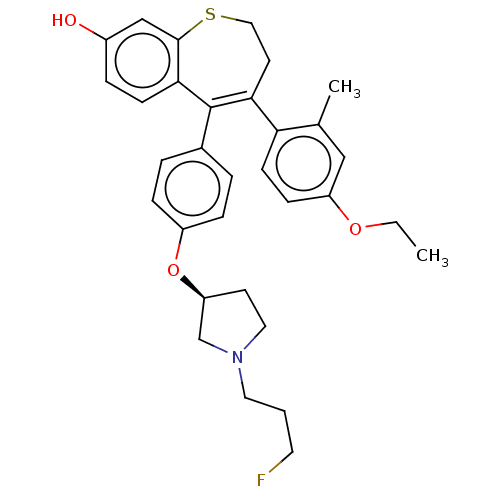 (4-(4-ethoxy-2- methyl-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521273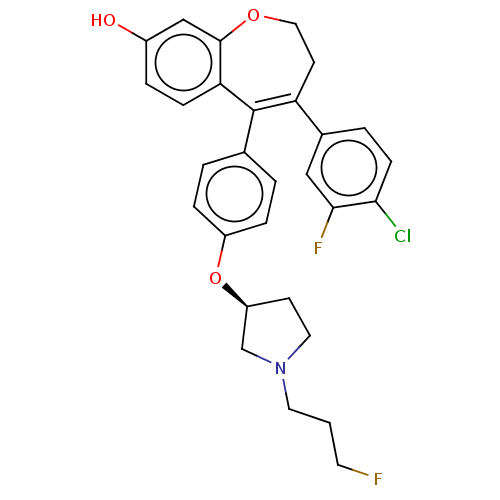 (4-(4-chloro-3- fluoro-phenyl)- 5-[4-[(3S)-1-(3- fl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521348 (5-[4-[(3S)-1-(3- fluoropropyl)pyr- rolidin-3- yl]o...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521297 (4-(2-fluoro-4- methoxy- phenyl)-5-[4- [(3S)-1-(3- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [298-554,Y537S] (Homo sapiens (Human)) | BDBM521197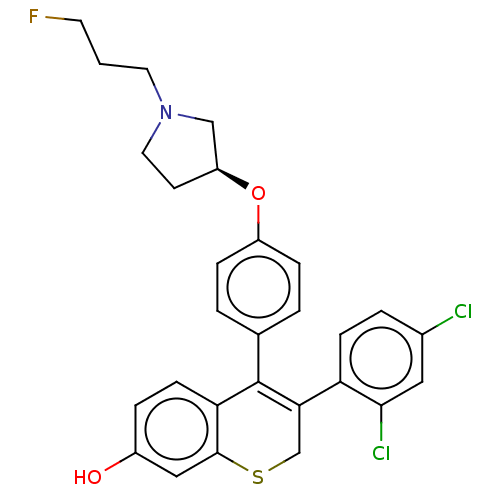 (3-(2,4- dichlorophenyl)- 4-[4-[(3S)-1-(3- fluoropr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonistic potency of compounds was evaluated using LanthaScreenŽ TR-FRET ERα Coactivator Assay (ThermoFisher) with modifications. It is a com... | Citation and Details BindingDB Entry DOI: 10.7270/Q2NZ8BT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 255 total ) | Next | Last >> |