

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236853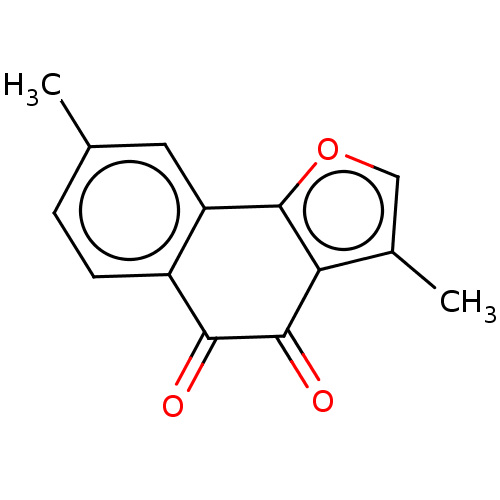 (CHEMBL4094677) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration required for antagonistic activity at Metabotropic glutamate receptor 2 | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236859 (CHEMBL4086536) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Substrate activity at CPR in human L02 cells assessed as CPR-mediated one-electron reduction of compound by measuring cell growth inhibition treated ... | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM81348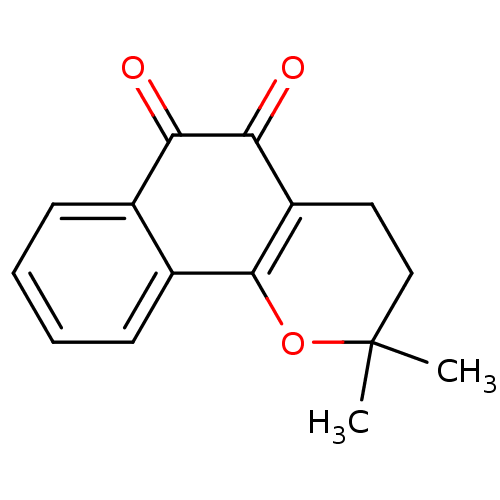 (β-Lapachone (A3) | Beta lapachone | R115 (Rea...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Substrate activity at CPR in human L02 cells assessed as CPR-mediated one-electron reduction of compound by measuring cell growth inhibition treated ... | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236854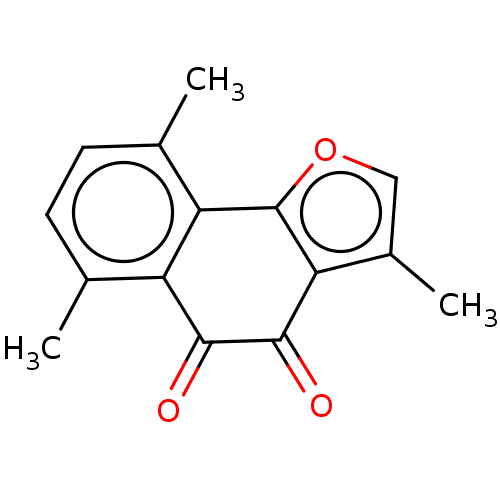 (CHEMBL4074173) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration required for antagonistic activity at Metabotropic glutamate receptor 2 | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236863 (CHEMBL3398294) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Substrate activity at CPR in human L02 cells assessed as CPR-mediated one-electron reduction of compound by measuring cell growth inhibition treated ... | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236858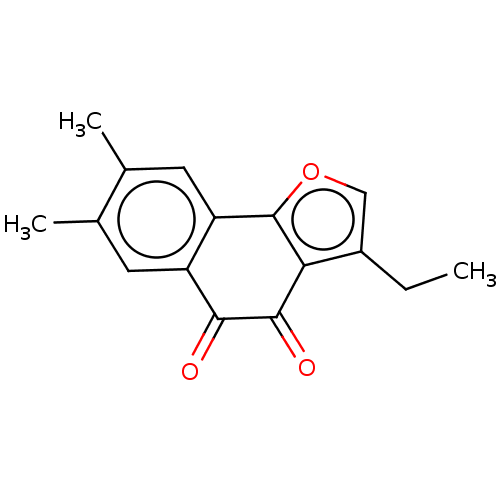 (CHEMBL4098063) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Functional potency measured as intracellular calcium elevation in Hek-293 cells expressing hGHSR1a | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236857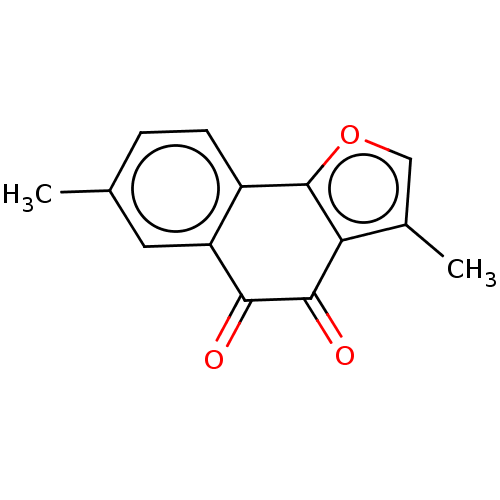 (CHEMBL4080037) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration required for antagonistic activity at Metabotropic glutamate receptor 2 | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236860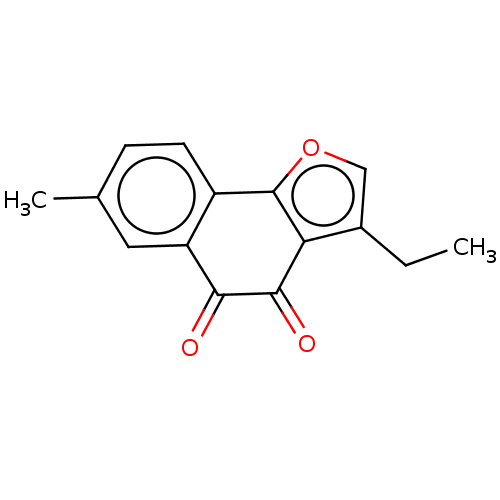 (CHEMBL4065098) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Functional potency measured as intracellular calcium elevation in Hek-293 cells expressing hGHSR1a | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236861 (CHEMBL3398292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Substrate activity at CPR in human L02 cells assessed as CPR-mediated one-electron reduction of compound by measuring cell growth inhibition treated ... | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236862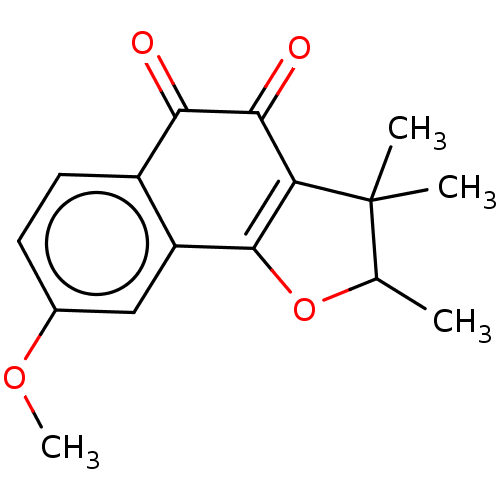 (CHEMBL3398293) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Substrate activity at CPR in human L02 cells assessed as CPR-mediated one-electron reduction of compound by measuring cell growth inhibition treated ... | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NADPH--cytochrome P450 reductase (Homo sapiens (Human)) | BDBM50236855 (CHEMBL4102425) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory concentration required for antagonistic activity at Metabotropic glutamate receptor 2; Maximum inhibition reached only 50% | Eur J Med Chem 129: 27-40 (2017) Article DOI: 10.1016/j.ejmech.2017.02.004 BindingDB Entry DOI: 10.7270/Q24170BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||