 Found 31 hits of ic50 for UniProtKB: P11086
Found 31 hits of ic50 for UniProtKB: P11086 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13014
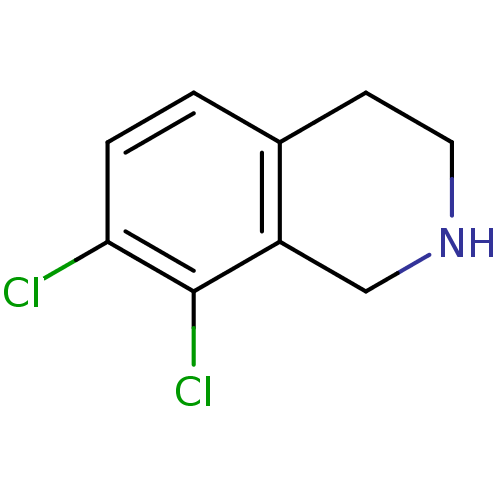
(7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...)Show InChI InChI=1S/C9H9Cl2N/c10-8-2-1-6-3-4-12-5-7(6)9(8)11/h1-2,12H,3-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PNMT |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13014

(7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...)Show InChI InChI=1S/C9H9Cl2N/c10-8-2-1-6-3-4-12-5-7(6)9(8)11/h1-2,12H,3-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13014

(7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...)Show InChI InChI=1S/C9H9Cl2N/c10-8-2-1-6-3-4-12-5-7(6)9(8)11/h1-2,12H,3-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50240934

(3-(hydroxymethyl)-N-(3,3,3-trifluoropropyl)-1,2,3,...)Show InChI InChI=1S/C13H17F3N2O3S/c14-13(15,16)3-4-18-22(20,21)12-2-1-9-5-11(8-19)17-7-10(9)6-12/h1-2,6,11,17-19H,3-5,7-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of PNMT |
J Med Chem 51: 3661-80 (2008)
Article DOI: 10.1021/jm8000373
BindingDB Entry DOI: 10.7270/Q2N58M4H |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028902

(7,8-dichloro-2-[2-(7,8-dichloro-1,2,3,4-tetrahydro...)Show SMILES Clc1ccc2CCN(CCN3CCc4ccc(Cl)c(Cl)c4C3)Cc2c1Cl Show InChI InChI=1S/C20H20Cl4N2/c21-17-3-1-13-5-7-25(11-15(13)19(17)23)9-10-26-8-6-14-2-4-18(22)20(24)16(14)12-26/h1-4H,5-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029108

(6,7,8-Trichloro-1,2,3,4-tetrahydro-isoquinoline | ...)Show InChI InChI=1S/C9H8Cl3N/c10-7-3-5-1-2-13-4-6(5)8(11)9(7)12/h3,13H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028908
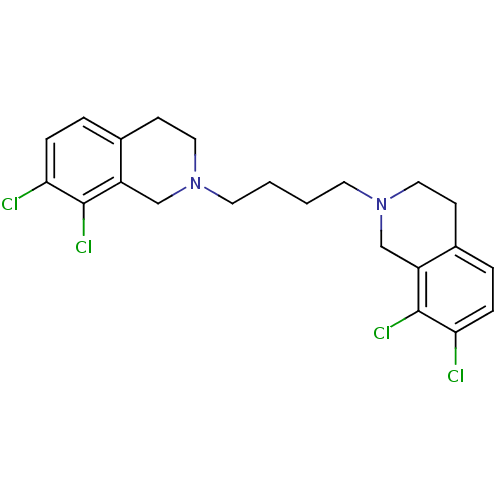
(7,8-dichloro-2-[4-(7,8-dichloro-1,2,3,4-tetrahydro...)Show SMILES Clc1ccc2CCN(CCCCN3CCc4ccc(Cl)c(Cl)c4C3)Cc2c1Cl Show InChI InChI=1S/C22H24Cl4N2/c23-19-5-3-15-7-11-27(13-17(15)21(19)25)9-1-2-10-28-12-8-16-4-6-20(24)22(26)18(16)14-28/h3-6H,1-2,7-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029107

(8-Chloro-1,2,3,4-tetrahydro-isoquinoline | CHEMBL1...)Show InChI InChI=1S/C9H10ClN/c10-9-3-1-2-7-4-5-11-6-8(7)9/h1-3,11H,4-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029102

(7-Chloro-1,2,3,4-tetrahydro-isoquinoline | CHEMBL1...)Show InChI InChI=1S/C9H10ClN/c10-9-2-1-7-3-4-11-6-8(7)5-9/h1-2,5,11H,3-4,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029101
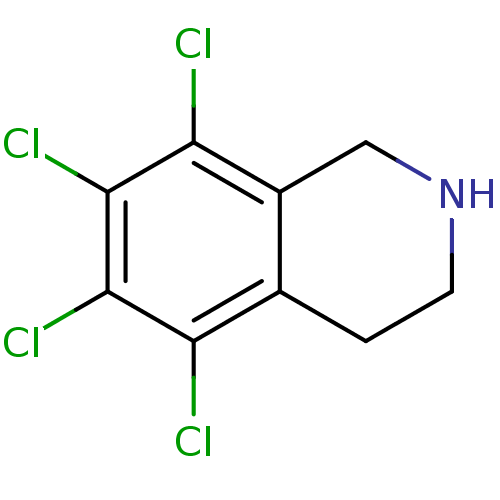
(5,6,7,8-Tetrachloro-1,2,3,4-tetrahydro-isoquinolin...)Show InChI InChI=1S/C9H7Cl4N/c10-6-4-1-2-14-3-5(4)7(11)9(13)8(6)12/h14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029099

(5,7,8-Trichloro-1,2,3,4-tetrahydro-isoquinoline | ...)Show InChI InChI=1S/C9H8Cl3N/c10-7-3-8(11)9(12)6-4-13-2-1-5(6)7/h3,13H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028903

(7,8-dichloro-2-[3-(7,8-dichloro-1,2,3,4-tetrahydro...)Show SMILES Clc1ccc2CCN(CCCN3CCc4ccc(Cl)c(Cl)c4C3)Cc2c1Cl Show InChI InChI=1S/C21H22Cl4N2/c22-18-4-2-14-6-10-26(12-16(14)20(18)24)8-1-9-27-11-7-15-3-5-19(23)21(25)17(15)13-27/h2-5H,1,6-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029106

(2,3-Dichloro-benzylamine | CHEMBL13165)Show InChI InChI=1S/C7H7Cl2N/c8-6-3-1-2-5(4-10)7(6)9/h1-3H,4,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028910
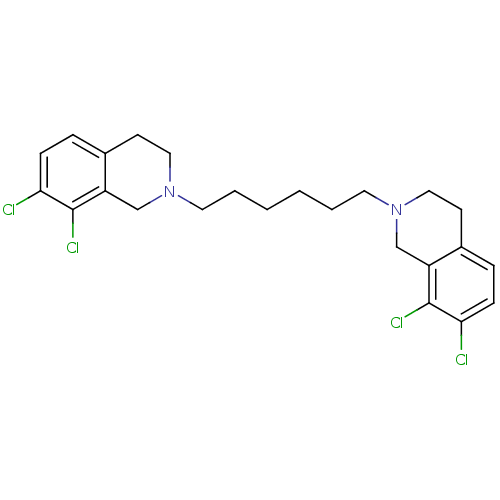
(7,8-dichloro-2-[6-(7,8-dichloro-1,2,3,4-tetrahydro...)Show SMILES Clc1ccc2CCN(CCCCCCN3CCc4ccc(Cl)c(Cl)c4C3)Cc2c1Cl Show InChI InChI=1S/C24H28Cl4N2/c25-21-7-5-17-9-13-29(15-19(17)23(21)27)11-3-1-2-4-12-30-14-10-18-6-8-22(26)24(28)20(18)16-30/h5-8H,1-4,9-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028901

(7,8-Dichloro-2-ethyl-1,2,3,4-tetrahydro-isoquinoli...)Show InChI InChI=1S/C11H13Cl2N/c1-2-14-6-5-8-3-4-10(12)11(13)9(8)7-14/h3-4H,2,5-7H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028914

(7,8-dichloro-2-[7-(7,8-dichloro-1,2,3,4-tetrahydro...)Show SMILES Clc1ccc2CCN(CCCCCCCN3CCc4ccc(Cl)c(Cl)c4C3)Cc2c1Cl Show InChI InChI=1S/C25H30Cl4N2/c26-22-8-6-18-10-14-30(16-20(18)24(22)28)12-4-2-1-3-5-13-31-15-11-19-7-9-23(27)25(29)21(19)17-31/h6-9H,1-5,10-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028911

(7,8-dichloro-2-[8-(7,8-dichloro-1,2,3,4-tetrahydro...)Show SMILES Clc1ccc2CCN(CCCCCCCCN3CCc4ccc(Cl)c(Cl)c4C3)Cc2c1Cl Show InChI InChI=1S/C26H32Cl4N2/c27-23-9-7-19-11-15-31(17-21(19)25(23)29)13-5-3-1-2-4-6-14-32-16-12-20-8-10-24(28)26(30)22(20)18-32/h7-10H,1-6,11-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028909
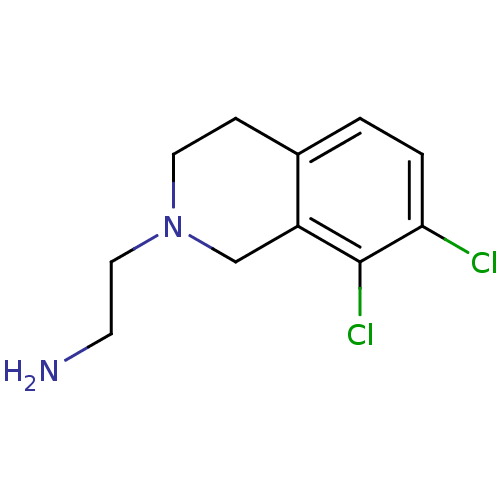
(2-(7,8-Dichloro-3,4-dihydro-1H-isoquinolin-2-yl)-e...)Show InChI InChI=1S/C11H14Cl2N2/c12-10-2-1-8-3-5-15(6-4-14)7-9(8)11(10)13/h1-2H,3-7,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028913

(7,8-Dichloro-2-propyl-1,2,3,4-tetrahydro-isoquinol...)Show InChI InChI=1S/C12H15Cl2N/c1-2-6-15-7-5-9-3-4-11(13)12(14)10(9)8-15/h3-4H,2,5-8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028912

(2-Benzyl-7,8-dichloro-1,2,3,4-tetrahydro-isoquinol...)Show InChI InChI=1S/C16H15Cl2N/c17-15-7-6-13-8-9-19(11-14(13)16(15)18)10-12-4-2-1-3-5-12/h1-7H,8-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM12584

((3-chlorophenyl)methanamine | 1-(3-CHLOROPHENYL)ME...)Show InChI InChI=1S/C7H8ClN/c8-7-3-1-2-6(4-7)5-9/h1-4H,5,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028906

(7,8-dichloro-2-[5-(7,8-dichloro-1,2,3,4-tetrahydro...)Show SMILES Clc1ccc2CCN(CCCCCN3CCc4ccc(Cl)c(Cl)c4C3)Cc2c1Cl Show InChI InChI=1S/C23H26Cl4N2/c24-20-6-4-16-8-12-28(14-18(16)22(20)26)10-2-1-3-11-29-13-9-17-5-7-21(25)23(27)19(17)15-29/h4-7H,1-3,8-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13014

(7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...)Show InChI InChI=1S/C9H9Cl2N/c10-8-2-1-6-3-4-12-5-7(6)9(8)11/h1-2,12H,3-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029104
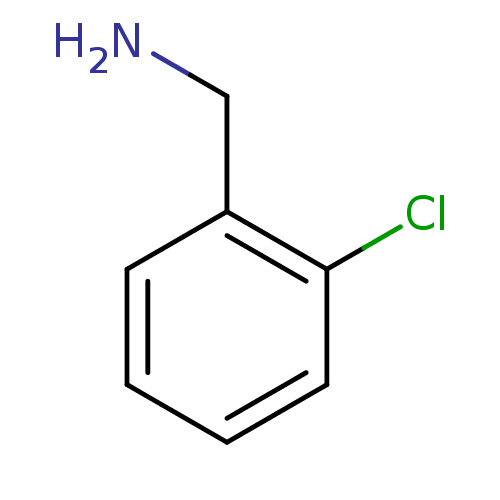
(2-Chloro-benzylamine | CHEMBL12712)Show InChI InChI=1S/C7H8ClN/c8-7-4-2-1-3-6(7)5-9/h1-4H,5,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029105
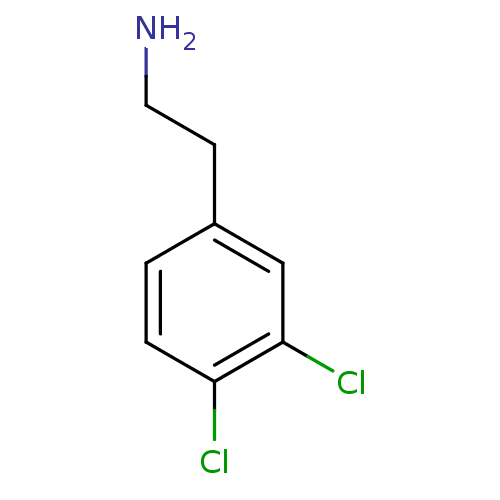
(2-(3,4,-dichlorophenyl)ethylamine | 2-(3,4-Dichlor...)Show InChI InChI=1S/C8H9Cl2N/c9-7-2-1-6(3-4-11)5-8(7)10/h1-2,5H,3-4,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028905
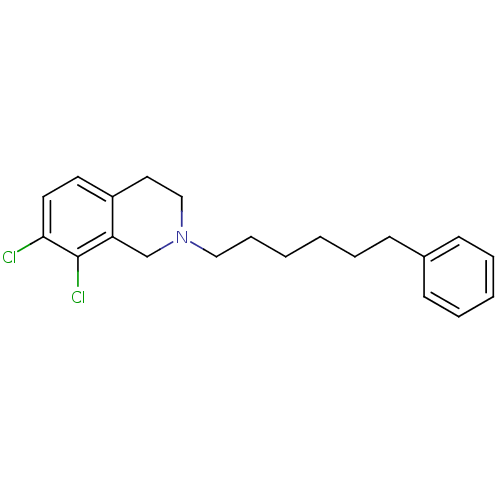
(7,8-Dichloro-2-(6-phenyl-hexyl)-1,2,3,4-tetrahydro...)Show InChI InChI=1S/C21H25Cl2N/c22-20-12-11-18-13-15-24(16-19(18)21(20)23)14-7-2-1-4-8-17-9-5-3-6-10-17/h3,5-6,9-12H,1-2,4,7-8,13-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029103

(2-(3-Chloro-phenyl)-1-methyl-ethylamine | CHEMBL14...)Show InChI InChI=1S/C9H12ClN/c1-7(11)5-8-3-2-4-9(10)6-8/h2-4,6-7H,5,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028907

(6-(7,8-Dichloro-3,4-dihydro-1H-isoquinolin-2-yl)-h...)Show InChI InChI=1S/C15H21Cl2NO/c16-14-6-5-12-7-9-18(11-13(12)15(14)17)8-3-1-2-4-10-19/h5-6,19H,1-4,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50506000

(CHEMBL4445337)Show SMILES N[C@@H](CCN(CC#Cc1cccc(c1)C(N)=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H28N8O6/c25-15(24(36)37)6-8-31(7-2-4-13-3-1-5-14(9-13)21(27)35)10-16-18(33)19(34)23(38-16)32-12-30-17-20(26)28-11-29-22(17)32/h1,3,5,9,11-12,15-16,18-19,23,33-34H,6-8,10,25H2,(H2,27,35)(H,36,37)(H2,26,28,29)/t15-,16+,18+,19+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6 tagged PNMT using norepinephrine as substrate preincubated for 10 mins in presence of AdoMet followed by substrat... |
J Med Chem 62: 10783-10797 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01255
BindingDB Entry DOI: 10.7270/Q2WD43VS |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50028904

(2-(6-Bromo-hexyl)-7,8-dichloro-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C15H20BrCl2N/c16-8-3-1-2-4-9-19-10-7-12-5-6-14(17)15(18)13(12)11-19/h5-6H,1-4,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rabbit adrenal phenylethanolamine N-methyl-transferase |
J Med Chem 24: 756-9 (1981)
BindingDB Entry DOI: 10.7270/Q2DZ08VH |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029100
(2-(4-Chloro-phenyl)-1-methyl-ethylamine | CHEMBL35...)Show InChI InChI=1S/C9H12ClN/c1-7(11)6-8-2-4-9(10)5-3-8/h2-5,7H,6,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data