 Found 27 hits of ki for UniProtKB: P49356
Found 27 hits of ki for UniProtKB: P49356 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50143597
((S)-2-(2-[2-(4-Fluoro-phenyl)-ethyl]-5-{[(1S,4S)-4...)Show SMILES CSCC[C@H](NC(=O)c1cc(NC[C@@H]2C[C@@H](CN2)SC(=O)c2cccnc2)ccc1CCc1ccc(F)cc1)C(=O)OC(C)C Show InChI InChI=1S/C34H41FN4O4S2/c1-22(2)43-33(41)31(14-16-44-3)39-32(40)30-18-27(13-10-24(30)9-6-23-7-11-26(35)12-8-23)37-20-28-17-29(21-38-28)45-34(42)25-5-4-15-36-19-25/h4-5,7-8,10-13,15,18-19,22,28-29,31,37-38H,6,9,14,16-17,20-21H2,1-3H3,(H,39,40)/t28-,29-,31-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition binding constant against Farnesyltransferase |
J Med Chem 47: 1869-78 (2004)
Article DOI: 10.1021/jm0305467
BindingDB Entry DOI: 10.7270/Q2RX9CVX |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50287709

((E)-3-Carboxy-2-(16-sulfooxy-hexadecyl)-pent-2-ene...)Show SMILES OC(=O)C\C(C(O)=O)=C(\CCCCCCCCCCCCCCCCOS(O)(=O)=O)C(O)=O Show InChI InChI=1S/C22H38O10S/c23-20(24)17-19(22(27)28)18(21(25)26)15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16-32-33(29,30)31/h1-17H2,(H,23,24)(H,25,26)(H,27,28)(H,29,30,31)/b19-18+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of human recombinant Protein farnesyltransferase with respect to FPP |
Bioorg Med Chem Lett 6: 2081-2084 (1996)
Article DOI: 10.1016/0960-894X(96)00372-1
BindingDB Entry DOI: 10.7270/Q2222TQ1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50492066
(CHEMBL2396761)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)CCCCCNC(=O)c1cc(OCCC(CN)CN)c(OCCC(CN)CN)c(OCCC(CN)CN)c1)C(C)C)C(=O)N[C@@H](CCSC)C(O)=O |r| Show InChI InChI=1S/C47H87N11O10S2/c1-6-30(4)41(46(63)56-35(47(64)65)14-19-70-5)58-45(62)40(29(2)3)57-44(61)36(28-69)55-39(59)10-8-7-9-15-54-43(60)34-20-37(66-16-11-31(22-48)23-49)42(68-18-13-33(26-52)27-53)38(21-34)67-17-12-32(24-50)25-51/h20-21,29-33,35-36,40-41,69H,6-19,22-28,48-53H2,1-5H3,(H,54,60)(H,55,59)(H,56,63)(H,57,61)(H,58,62)(H,64,65)/t30-,35-,36-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by microplate reader analysis |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50059860
(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl pyrophosphate |
J Med Chem 40: 2971-90 (1997)
Article DOI: 10.1021/jm970226l
BindingDB Entry DOI: 10.7270/Q26M37HF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50492066

(CHEMBL2396761)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)CCCCCNC(=O)c1cc(OCCC(CN)CN)c(OCCC(CN)CN)c(OCCC(CN)CN)c1)C(C)C)C(=O)N[C@@H](CCSC)C(O)=O |r| Show InChI InChI=1S/C47H87N11O10S2/c1-6-30(4)41(46(63)56-35(47(64)65)14-19-70-5)58-45(62)40(29(2)3)57-44(61)36(28-69)55-39(59)10-8-7-9-15-54-43(60)34-20-37(66-16-11-31(22-48)23-49)42(68-18-13-33(26-52)27-53)38(21-34)67-17-12-32(24-50)25-51/h20-21,29-33,35-36,40-41,69H,6-19,22-28,48-53H2,1-5H3,(H,54,60)(H,55,59)(H,56,63)(H,57,61)(H,58,62)(H,64,65)/t30-,35-,36-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by fluorescence plate reader analy... |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088001
((+/-)-11-[2-(2-methoxyphenyl)acryloyl]-1-phenyl-(1...)Show SMILES COc1ccccc1C(=C)C(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2ccccc12)C(O)=O |THB:28:27:15.14:19.18,31:32:15.14:19.18| Show InChI InChI=1S/C31H29NO4/c1-20(22-12-7-9-15-26(22)36-2)28(33)32-18-27-30(21-10-4-3-5-11-21)17-16-25(31(27,19-32)29(34)35)23-13-6-8-14-24(23)30/h3-15,25,27H,1,16-19H2,2H3,(H,34,35)/t25-,27+,30-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088009

((+/-)-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2ccccc12)C(O)=O |THB:30:31:14.13:18.17,27:26:14.13:18.17| Show InChI InChI=1S/C30H29NO4/c1-35-25-14-8-5-9-20(25)17-27(32)31-18-26-29(21-10-3-2-4-11-21)16-15-24(30(26,19-31)28(33)34)22-12-6-7-13-23(22)29/h2-14,24,26H,15-19H2,1H3,(H,33,34)/t24-,26+,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50289150

(Acetic acid (7E,11E)-(1S,2R,4R,14S,15R)-4,8,12-tri...)Show SMILES CC(=O)O[C@H]1C\C(C)=C\CC\C(C)=C\CC[C@@]2(C)O[C@@H]2[C@H]2OC(=O)C(=C)[C@H]12 |t:7,12| Show InChI InChI=1S/C22H30O5/c1-13-8-6-9-14(2)12-17(25-16(4)23)18-15(3)21(24)26-19(18)20-22(5,27-20)11-7-10-13/h9-10,17-20H,3,6-8,11-12H2,1-2,4-5H3/b13-10+,14-9+/t17-,18+,19-,20+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Kinetic parameter for inihibiton of farnesyl protein transferase |
Bioorg Med Chem Lett 6: 909-912 (1996)
Article DOI: 10.1016/0960-894X(96)00142-4
BindingDB Entry DOI: 10.7270/Q2VT1S3W |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481616
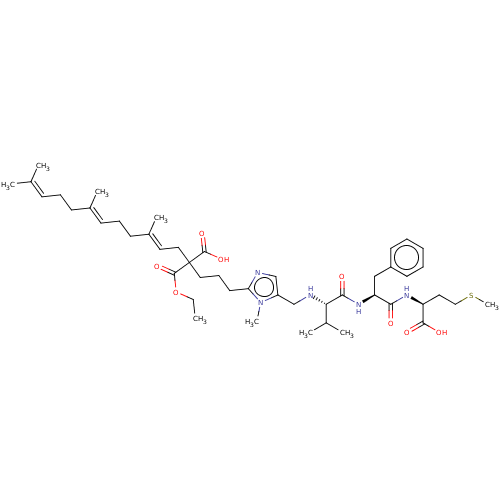
(CHEMBL590127)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]-[#6]-[#6]-c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H71N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,17,19,21-22,24,30,33,38-39,41,49H,10,14-16,18,20,23,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088004
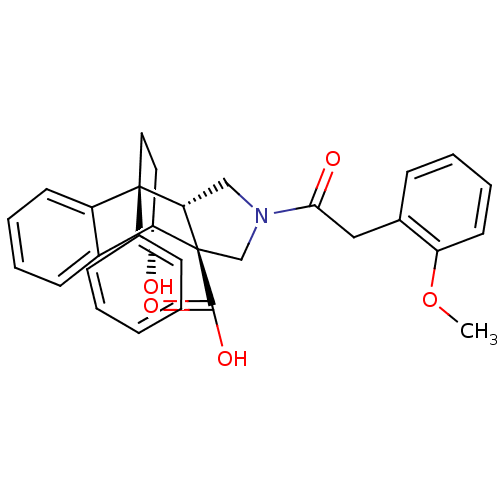
(8-hydroxy-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@@](C1)(C(O)=O)[C@]1(O)CC[C@@]2(c2ccccc2)c2ccccc12 Show InChI InChI=1S/C30H29NO5/c1-36-24-14-8-5-9-20(24)17-26(32)31-18-25-28(21-10-3-2-4-11-21)15-16-30(35,29(25,19-31)27(33)34)23-13-7-6-12-22(23)28/h2-14,25,35H,15-19H2,1H3,(H,33,34)/t25-,28+,29-,30-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481625

(CHEMBL599795)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#6](-[#6](-[#8])=O)-[#6](-[#8])=O Show InChI InChI=1S/C18H28O4/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-16(17(19)20)18(21)22/h7,9,11,16H,5-6,8,10,12H2,1-4H3,(H,19,20)(H,21,22)/b14-9+,15-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088008
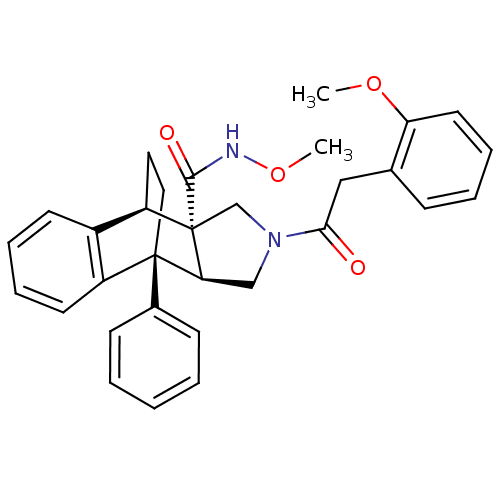
(11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,8R,9S,...)Show SMILES CONC(=O)[C@]12CN(C[C@H]1[C@]1(CC[C@@H]2c2ccccc12)c1ccccc1)C(=O)Cc1ccccc1OC |THB:15:14:5.9:11.12,18:19:5.9:11.12| Show InChI InChI=1S/C31H32N2O4/c1-36-26-15-9-6-10-21(26)18-28(34)33-19-27-30(22-11-4-3-5-12-22)17-16-25(23-13-7-8-14-24(23)30)31(27,20-33)29(35)32-37-2/h3-15,25,27H,16-20H2,1-2H3,(H,32,35)/t25-,27+,30-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088014

(1-(2,3-dihydrobenzo[b]furan-5-yl)-11-[2-(2-methoxy...)Show SMILES COc1ccccc1C(=C)C(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccc3OCCc3c2)c2ccccc12)C(O)=O |THB:31:30:15.14:19.18,34:35:15.14:19.18| Show InChI InChI=1S/C33H31NO5/c1-20(23-7-4-6-10-28(23)38-2)30(35)34-18-29-32(22-11-12-27-21(17-22)14-16-39-27)15-13-26(33(29,19-34)31(36)37)24-8-3-5-9-25(24)32/h3-12,17,26,29H,1,13-16,18-19H2,2H3,(H,36,37)/t26-,29+,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481615

(CHEMBL590126)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]\[#6]=[#6]\c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H69N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,16-17,19,21-24,30,33,38-39,41,49H,10,14-15,18,20,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b23-16+,34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to FPP site of human recombinant FTase by competitive Michaelis-Menten analysis |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088013
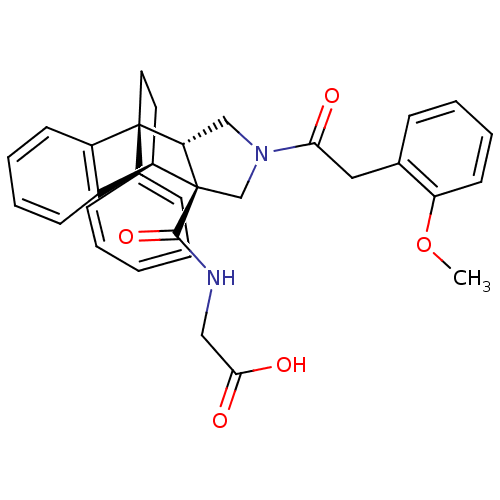
(2-[11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-(1R,8R,...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2ccccc12)C(=O)NCC(O)=O |THB:27:26:14.13:18.17,30:31:14.13:18.17| Show InChI InChI=1S/C32H32N2O5/c1-39-26-14-8-5-9-21(26)17-28(35)34-19-27-31(22-10-3-2-4-11-22)16-15-25(23-12-6-7-13-24(23)31)32(27,20-34)30(38)33-18-29(36)37/h2-14,25,27H,15-20H2,1H3,(H,33,38)(H,36,37)/t25-,27+,31-,32+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 738 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50108070

(2-[4-(2-Amino-3-mercapto-propylamino)-benzoylamino...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@@H](N)CS)cc1)C(O)=O Show InChI InChI=1S/C15H23N3O3S2/c1-23-7-6-13(15(20)21)18-14(19)10-2-4-12(5-3-10)17-8-11(16)9-22/h2-5,11,13,17,22H,6-9,16H2,1H3,(H,18,19)(H,20,21)/t11-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 785 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by fluorescence plate reader analy... |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50059865
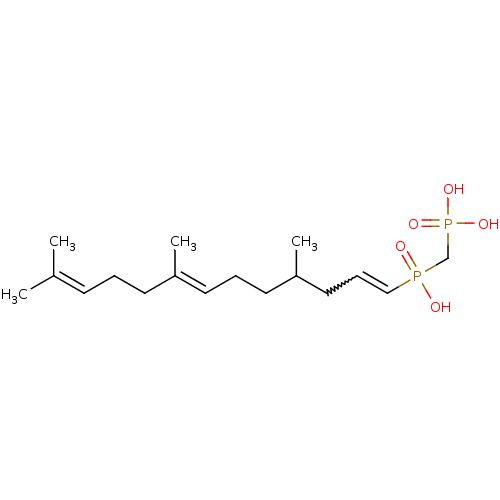
(4,8,12-trimethyl-(3E,7E)-3,7,11-tridecatrienylhydr...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6]=[#6]P([#8])(=O)[#6]P([#8])([#8])=O |w:14.13| Show InChI InChI=1S/C17H32O5P2/c1-15(2)8-5-9-16(3)10-6-11-17(4)12-7-13-23(18,19)14-24(20,21)22/h7-8,10,13,17H,5-6,9,11-12,14H2,1-4H3,(H,18,19)(H2,20,21,22)/b13-7?,16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against farnesyl pyrophosphate |
J Med Chem 40: 2971-90 (1997)
Article DOI: 10.1021/jm970226l
BindingDB Entry DOI: 10.7270/Q26M37HF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50492065
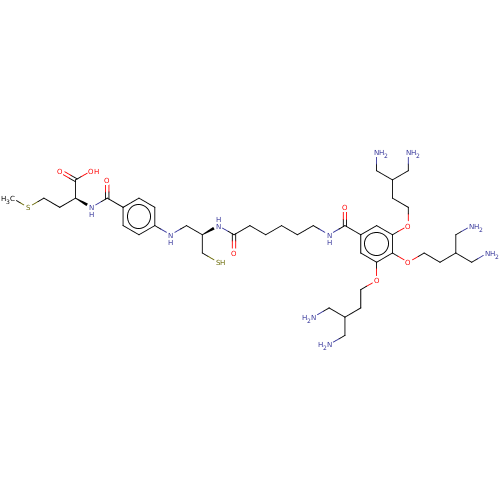
(CHEMBL2396762)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@H](CS)NC(=O)CCCCCNC(=O)c2cc(OCCC(CN)CN)c(OCCC(CN)CN)c(OCCC(CN)CN)c2)cc1)C(O)=O |r| Show InChI InChI=1S/C43H74N10O8S2/c1-63-18-13-36(43(57)58)53-42(56)32-6-8-34(9-7-32)51-27-35(28-62)52-39(54)5-3-2-4-14-50-41(55)33-19-37(59-15-10-29(21-44)22-45)40(61-17-12-31(25-48)26-49)38(20-33)60-16-11-30(23-46)24-47/h6-9,19-20,29-31,35-36,51,62H,2-5,10-18,21-28,44-49H2,1H3,(H,50,55)(H,52,54)(H,53,56)(H,57,58)/t35-,36+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 837 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by fluorescence plate reader analy... |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50108076
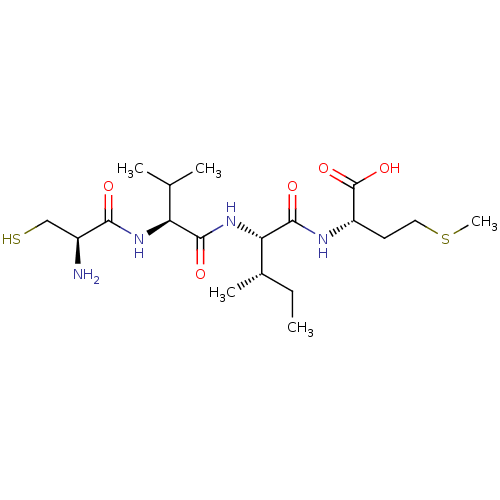
((S)-2-{(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propio...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CS)C(C)C)C(=O)N[C@@H](CCSC)C(O)=O Show InChI InChI=1S/C19H36N4O5S2/c1-6-11(4)15(18(26)21-13(19(27)28)7-8-30-5)23-17(25)14(10(2)3)22-16(24)12(20)9-29/h10-15,29H,6-9,20H2,1-5H3,(H,21,26)(H,22,24)(H,23,25)(H,27,28)/t11-,12-,13-,14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Inhibition of FTase (unknown origin)-mediated K-Ras4B model peptide KKKKKKSK(Dans)TKCVIM farnesylation by microplate reader analysis |
Bioorg Med Chem 21: 4004-10 (2013)
Article DOI: 10.1016/j.bmc.2012.09.061
BindingDB Entry DOI: 10.7270/Q2ZS30GN |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481616

(CHEMBL590127)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]-[#6]-[#6]-c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H71N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,17,19,21-22,24,30,33,38-39,41,49H,10,14-16,18,20,23,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-substrate-inhibitor complex |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481616

(CHEMBL590127)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]-[#6]-[#6]-c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H71N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,17,19,21-22,24,30,33,38-39,41,49H,10,14-16,18,20,23,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-inhibitor complex |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481615

(CHEMBL590126)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]\[#6]=[#6]\c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H69N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,16-17,19,21-24,30,33,38-39,41,49H,10,14-15,18,20,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b23-16+,34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-substrate-inhibitor complex |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50481615

(CHEMBL590126)Show SMILES [#6]-[#6]-[#8]-[#6](=O)C([#6]\[#6]=[#6]\c1ncc(-[#6]-[#7]-[#6@@H](-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-c2ccccc2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)n1-[#6])([#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6](-[#8])=O |r| Show InChI InChI=1S/C47H69N5O8S/c1-10-60-46(59)47(45(57)58,27-24-35(7)20-15-19-34(6)18-14-17-32(2)3)26-16-23-40-48-30-37(52(40)8)31-49-41(33(4)5)43(54)51-39(29-36-21-12-11-13-22-36)42(53)50-38(44(55)56)25-28-61-9/h11-13,16-17,19,21-24,30,33,38-39,41,49H,10,14-15,18,20,25-29,31H2,1-9H3,(H,50,53)(H,51,54)(H,55,56)(H,57,58)/b23-16+,34-19+,35-24+/t38-,39-,41-,47?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Gif-sur-Yvette
Curated by ChEMBL
| Assay Description
Binding affinity to CaaX site of human recombinant FTase by non-competitive Michaelis-Menten analysis for enzyme-inhibitor complex |
Bioorg Med Chem 18: 543-56 (2010)
Article DOI: 10.1016/j.bmc.2009.12.017
BindingDB Entry DOI: 10.7270/Q2VM4G37 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088002

(1-(4-carboxymethoxyphenyl)-11-[2-(2-methoxyphenyl)...)Show SMILES COc1ccccc1C(=C)C(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccc(OCC(O)=O)cc2)c2ccccc12)C(O)=O |THB:36:37:15.14:19.18,33:32:15.14:19.18| Show InChI InChI=1S/C33H31NO7/c1-20(23-7-4-6-10-27(23)40-2)30(37)34-17-28-32(21-11-13-22(14-12-21)41-18-29(35)36)16-15-26(33(28,19-34)31(38)39)24-8-3-5-9-25(24)32/h3-14,26,28H,1,15-19H2,2H3,(H,35,36)(H,38,39)/t26-,28+,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088003
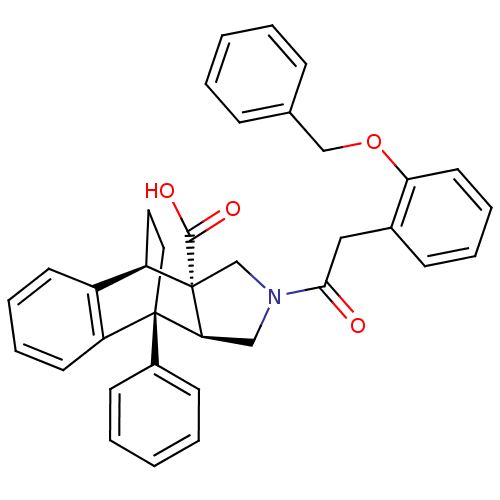
(11-[2-(2-benzyloxyphenyl)acetyl]-1-phenyl-(1R,8R,9...)Show SMILES OC(=O)[C@]12CN(C[C@H]1[C@]1(CC[C@@H]2c2ccccc12)c1ccccc1)C(=O)Cc1ccccc1OCc1ccccc1 |THB:13:12:3.7:9.10,16:17:3.7:9.10| Show InChI InChI=1S/C36H33NO4/c38-33(21-26-13-7-10-18-31(26)41-23-25-11-3-1-4-12-25)37-22-32-35(27-14-5-2-6-15-27)20-19-30(36(32,24-37)34(39)40)28-16-8-9-17-29(28)35/h1-18,30,32H,19-24H2,(H,39,40)/t30-,32+,35-,36+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088005
(2-(2-methoxyphenyl)-1-[1-phenyl-(1R,8S,9R,13R)-11-...)Show SMILES COc1ccccc1CC(=O)N1C[C@H]2[C@@H](C1)[C@]1(CC[C@@H]2c2ccccc12)c1ccccc1 Show InChI InChI=1S/C29H29NO2/c1-32-27-14-8-5-9-20(27)17-28(31)30-18-24-22-15-16-29(26(24)19-30,21-10-3-2-4-11-21)25-13-7-6-12-23(22)25/h2-14,22,24,26H,15-19H2,1H3/t22-,24-,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50088006

(6-hydroxy-11-[2-(2-methoxyphenyl)acetyl]-1-phenyl-...)Show SMILES COc1ccccc1CC(=O)N1C[C@@H]2[C@](C1)([C@@H]1CC[C@@]2(c2ccccc2)c2cccc(O)c12)C(O)=O |THB:30:32:14.13:18.17,27:26:14.13:18.17| Show InChI InChI=1S/C30H29NO5/c1-36-24-13-6-5-8-19(24)16-26(33)31-17-25-29(20-9-3-2-4-10-20)15-14-22(30(25,18-31)28(34)35)27-21(29)11-7-12-23(27)32/h2-13,22,25,32H,14-18H2,1H3,(H,34,35)/t22-,25+,29-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer S.A.
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase by Ras/SPA assay |
J Med Chem 43: 1807-16 (2000)
BindingDB Entry DOI: 10.7270/Q23N22MR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data