 Found 182 hits of ki for UniProtKB: P00797
Found 182 hits of ki for UniProtKB: P00797 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50025935
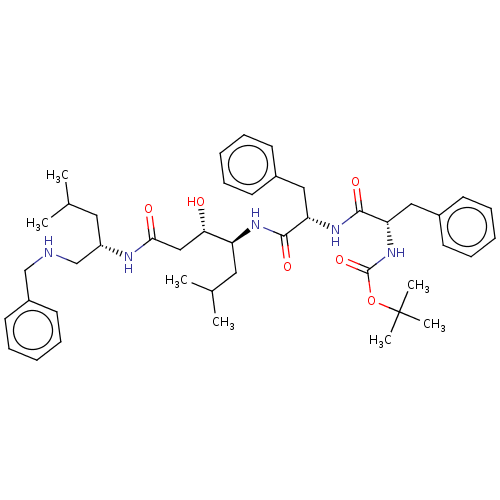
(CHEMBL3144404 | {1-[1-(1-{2-[1-(Benzylamino-methyl...)Show SMILES CC(C)C[C@@H](CNCc1ccccc1)NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C44H63N5O6/c1-30(2)23-35(29-45-28-34-21-15-10-16-22-34)46-40(51)27-39(50)36(24-31(3)4)47-41(52)37(25-32-17-11-8-12-18-32)48-42(53)38(26-33-19-13-9-14-20-33)49-43(54)55-44(5,6)7/h8-22,30-31,35-39,45,50H,23-29H2,1-7H3,(H,46,51)(H,47,52)(H,48,53)(H,49,54) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of hog kidney renin |
J Med Chem 28: 1755-6 (1986)
BindingDB Entry DOI: 10.7270/Q2P26X56 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023090
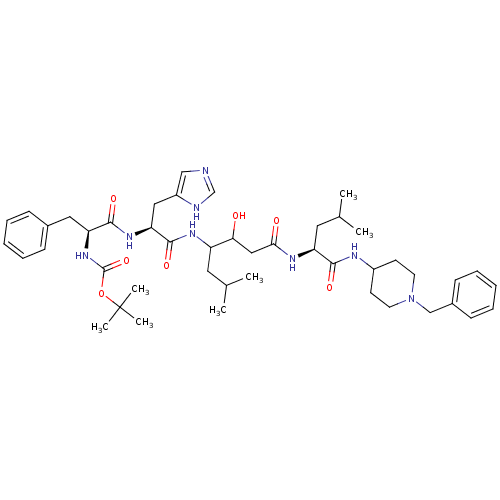
(Boc-Phe-His-Sta-Leu-4-amido-1-benzylpiperidine | C...)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)NC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C46H68N8O7/c1-30(2)22-36(40(55)26-41(56)50-37(23-31(3)4)42(57)49-34-18-20-54(21-19-34)28-33-16-12-9-13-17-33)51-44(59)39(25-35-27-47-29-48-35)52-43(58)38(24-32-14-10-8-11-15-32)53-45(60)61-46(5,6)7/h8-17,27,29-31,34,36-40,55H,18-26,28H2,1-7H3,(H,47,48)(H,49,57)(H,50,56)(H,51,59)(H,52,58)(H,53,60)/t36?,37-,38-,39-,40?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human kidney renin, radioimmunoassay using a synthetic tetradecapeptide renin substrate at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50368278
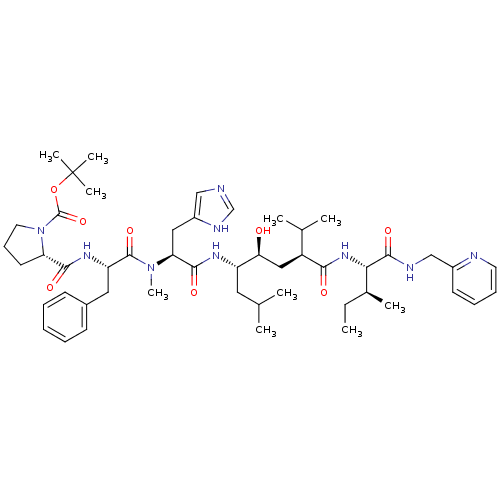
(CHEMBL1790497)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C50H75N9O8/c1-11-33(6)43(47(64)53-29-35-20-15-16-22-52-35)57-44(61)37(32(4)5)27-42(60)38(24-31(2)3)55-46(63)41(26-36-28-51-30-54-36)58(10)48(65)39(25-34-18-13-12-14-19-34)56-45(62)40-21-17-23-59(40)49(66)67-50(7,8)9/h12-16,18-20,22,28,30-33,37-43,60H,11,17,21,23-27,29H2,1-10H3,(H,51,54)(H,53,64)(H,55,63)(H,56,62)(H,57,61)/t33-,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition constants using recombinant human renin assay |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50368274
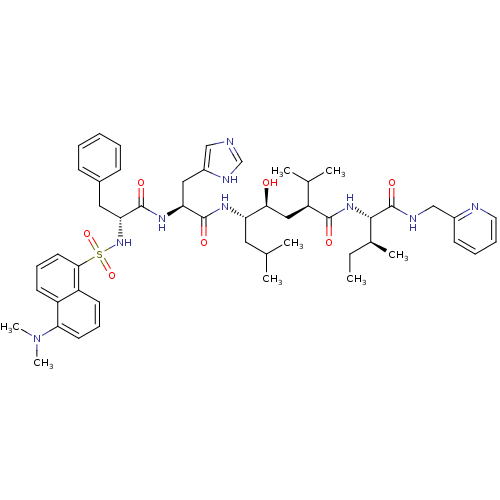
(CHEMBL1790492)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](Cc1ccccc1)NS(=O)(=O)c1cccc2c(cccc12)N(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C51H69N9O7S/c1-9-34(6)47(51(65)54-30-36-19-13-14-24-53-36)58-48(62)40(33(4)5)28-45(61)41(25-32(2)3)56-49(63)42(27-37-29-52-31-55-37)57-50(64)43(26-35-17-11-10-12-18-35)59-68(66,67)46-23-16-20-38-39(46)21-15-22-44(38)60(7)8/h10-24,29,31-34,40-43,45,47,59,61H,9,25-28,30H2,1-8H3,(H,52,55)(H,54,65)(H,56,63)(H,57,64)(H,58,62)/t34-,40-,41-,42-,43+,45-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition constants using recombinant human renin assay |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50012322

(CHEMBL264524 | N-(2-Hydroxy-1,1-bis-hydroxymethyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)N(C)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)NC(CO)(CO)CO)C(C)C)C(=O)NCc1cccc[n+]1[O-] Show InChI InChI=1S/C50H76N10O11/c1-8-33(6)43(47(68)52-26-36-17-12-13-20-60(36)71)56-44(65)37(32(4)5)24-42(64)38(21-31(2)3)54-46(67)41(23-35-25-51-30-53-35)58(7)48(69)39(22-34-15-10-9-11-16-34)55-45(66)40-18-14-19-59(40)49(70)57-50(27-61,28-62)29-63/h9-13,15-17,20,25,30-33,37-43,61-64H,8,14,18-19,21-24,26-29H2,1-7H3,(H,51,53)(H,52,68)(H,54,67)(H,55,66)(H,56,65)(H,57,70)/t33?,37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human renin by radio-immuno assay of angiotensin I (ANG I) |
J Med Chem 34: 2107-12 (1991)
BindingDB Entry DOI: 10.7270/Q2HQ40J6 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025934
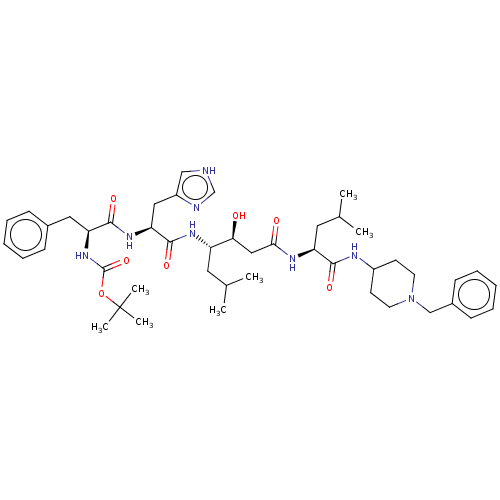
(CHEMBL3144405 | {1-[1-(1-{2-[1-(1-Benzyl-piperidin...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)NC1CCN(Cc2ccccc2)CC1 |r| Show InChI InChI=1S/C46H68N8O7/c1-30(2)22-36(40(55)26-41(56)50-37(23-31(3)4)42(57)49-34-18-20-54(21-19-34)28-33-16-12-9-13-17-33)51-44(59)39(25-35-27-47-29-48-35)52-43(58)38(24-32-14-10-8-11-15-32)53-45(60)61-46(5,6)7/h8-17,27,29-31,34,36-40,55H,18-26,28H2,1-7H3,(H,47,48)(H,49,57)(H,50,56)(H,51,59)(H,52,58)(H,53,60) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human kidney renin |
J Med Chem 28: 1755-6 (1986)
BindingDB Entry DOI: 10.7270/Q2P26X56 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023090

(Boc-Phe-His-Sta-Leu-4-amido-1-benzylpiperidine | C...)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)NC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C46H68N8O7/c1-30(2)22-36(40(55)26-41(56)50-37(23-31(3)4)42(57)49-34-18-20-54(21-19-34)28-33-16-12-9-13-17-33)51-44(59)39(25-35-27-47-29-48-35)52-43(58)38(24-32-14-10-8-11-15-32)53-45(60)61-46(5,6)7/h8-17,27,29-31,34,36-40,55H,18-26,28H2,1-7H3,(H,47,48)(H,49,57)(H,50,56)(H,51,59)(H,52,58)(H,53,60)/t36?,37-,38-,39-,40?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human kidney renin, fluorometric assay using a synthetic tetradecapeptide renin substrate at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023094
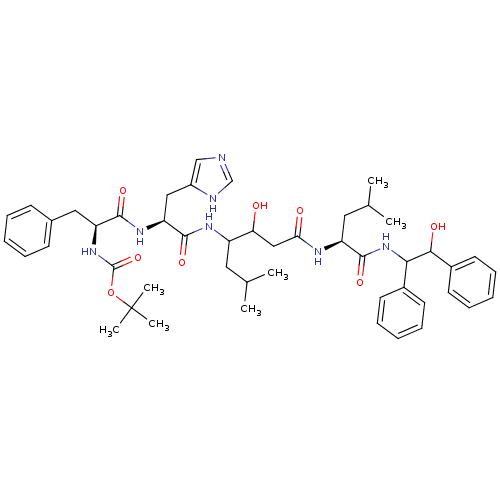
(Boc-Phe-His-Sta-Leu-erythro-1,2-diphenyl-2-hydroxy...)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)NC(C(O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C48H65N7O8/c1-30(2)23-36(40(56)27-41(57)51-37(24-31(3)4)46(61)55-42(33-19-13-9-14-20-33)43(58)34-21-15-10-16-22-34)52-45(60)39(26-35-28-49-29-50-35)53-44(59)38(25-32-17-11-8-12-18-32)54-47(62)63-48(5,6)7/h8-22,28-31,36-40,42-43,56,58H,23-27H2,1-7H3,(H,49,50)(H,51,57)(H,52,60)(H,53,59)(H,54,62)(H,55,61)/t36?,37-,38-,39-,40?,42?,43?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of hog kidney renin at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190
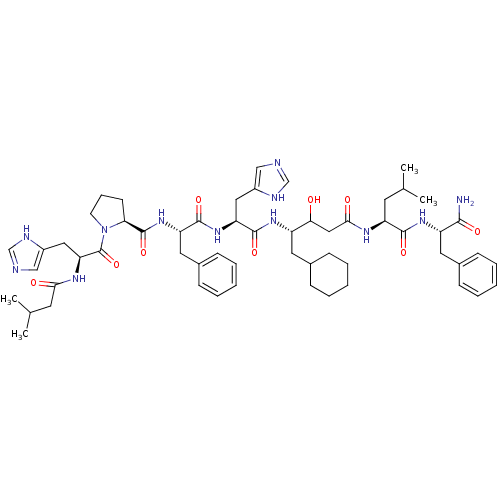
(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190

(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of rhesus monkey plasma renin. |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023094

(Boc-Phe-His-Sta-Leu-erythro-1,2-diphenyl-2-hydroxy...)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)NC(C(O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C48H65N7O8/c1-30(2)23-36(40(56)27-41(57)51-37(24-31(3)4)46(61)55-42(33-19-13-9-14-20-33)43(58)34-21-15-10-16-22-34)52-45(60)39(26-35-28-49-29-50-35)53-44(59)38(25-32-17-11-8-12-18-32)54-47(62)63-48(5,6)7/h8-22,28-31,36-40,42-43,56,58H,23-27H2,1-7H3,(H,49,50)(H,51,57)(H,52,60)(H,53,59)(H,54,62)(H,55,61)/t36?,37-,38-,39-,40?,42?,43?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human kidney renin, radioimmunoassay using the natural substrate partially pure angiotensinogen at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023094

(Boc-Phe-His-Sta-Leu-erythro-1,2-diphenyl-2-hydroxy...)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)NC(C(O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C48H65N7O8/c1-30(2)23-36(40(56)27-41(57)51-37(24-31(3)4)46(61)55-42(33-19-13-9-14-20-33)43(58)34-21-15-10-16-22-34)52-45(60)39(26-35-28-49-29-50-35)53-44(59)38(25-32-17-11-8-12-18-32)54-47(62)63-48(5,6)7/h8-22,28-31,36-40,42-43,56,58H,23-27H2,1-7H3,(H,49,50)(H,51,57)(H,52,60)(H,53,59)(H,54,62)(H,55,61)/t36?,37-,38-,39-,40?,42?,43?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human kidney renin, radioimmunoassay using a synthetic tetradecapeptide renin substrate at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025937
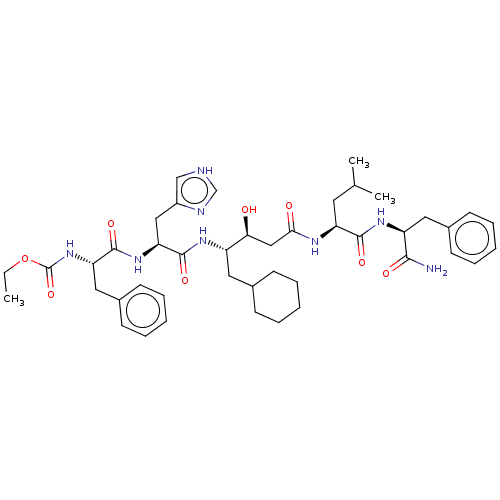
(CHEMBL3144417 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...)Show SMILES CCOC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C44H62N8O8/c1-4-60-44(59)52-36(23-31-18-12-7-13-19-31)42(57)51-37(24-32-26-46-27-47-32)43(58)49-33(21-29-14-8-5-9-15-29)38(53)25-39(54)48-35(20-28(2)3)41(56)50-34(40(45)55)22-30-16-10-6-11-17-30/h6-7,10-13,16-19,26-29,33-38,53H,4-5,8-9,14-15,20-25H2,1-3H3,(H2,45,55)(H,46,47)(H,48,54)(H,49,58)(H,50,56)(H,51,57)(H,52,59) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190

(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065428
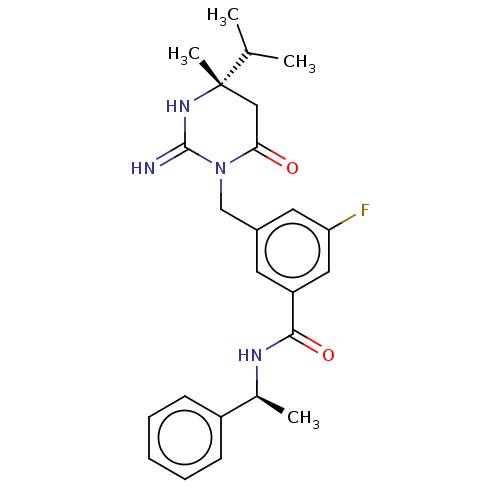
(CHEMBL3401350)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cc(F)cc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29FN4O2/c1-15(2)24(4)13-21(30)29(23(26)28-24)14-17-10-19(12-20(25)11-17)22(31)27-16(3)18-8-6-5-7-9-18/h5-12,15-16H,13-14H2,1-4H3,(H2,26,28)(H,27,31)/t16-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50077669

((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)C1CC1 Show InChI InChI=1S/C33H50N4O6S/c1-33(2,3)44(42,43)20-25(16-22-10-6-4-7-11-22)31(40)37-28(18-26-19-34-21-35-26)32(41)36-27(17-23-12-8-5-9-13-23)30(39)29(38)24-14-15-24/h4,6-7,10-11,19,21,23-25,27-30,38-39H,5,8-9,12-18,20H2,1-3H3,(H,34,35)(H,36,41)(H,37,40)/t25-,27+,28+,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic protease renin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17941
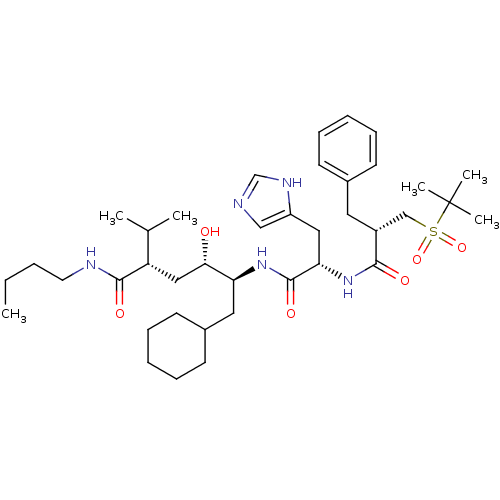
((2S,4S,5S)-5-[(2S)-2-[(2S)-2-benzyl-3-[(2-methylpr...)Show SMILES CCCCNC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C |r| Show InChI InChI=1S/C39H63N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-35,45H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46)/t30-,32+,33+,34+,35+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic protease renin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50025945
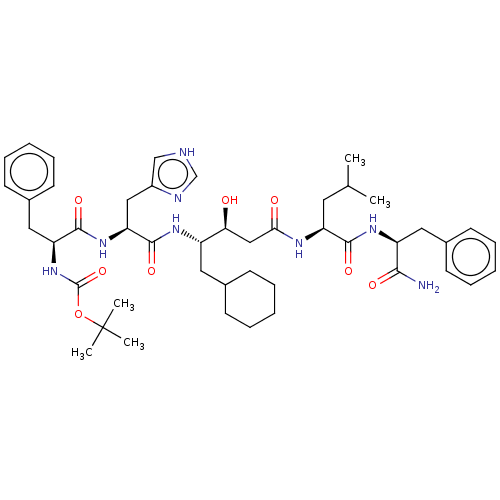
(CHEMBL3144416 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C46H66N8O8/c1-29(2)21-36(42(58)52-35(41(47)57)23-31-17-11-7-12-18-31)50-40(56)26-39(55)34(22-30-15-9-6-10-16-30)51-44(60)38(25-33-27-48-28-49-33)53-43(59)37(24-32-19-13-8-14-20-32)54-45(61)62-46(3,4)5/h7-8,11-14,17-20,27-30,34-39,55H,6,9-10,15-16,21-26H2,1-5H3,(H2,47,57)(H,48,49)(H,50,56)(H,51,60)(H,52,58)(H,53,59)(H,54,61) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human kidney renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50027362

(1-[2-(Hydroxy-phenoxy-phosphorylamino)-propionyl]-...)Show SMILES C[C@H](NP(O)(=O)Oc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C14H19N2O6P/c1-10(13(17)16-9-5-8-12(16)14(18)19)15-23(20,21)22-11-6-3-2-4-7-11/h2-4,6-7,10,12H,5,8-9H2,1H3,(H,18,19)(H2,15,20,21)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antihypertensive activity against human renin |
J Med Chem 24: 355-61 (1981)
BindingDB Entry DOI: 10.7270/Q2PC32XP |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025941
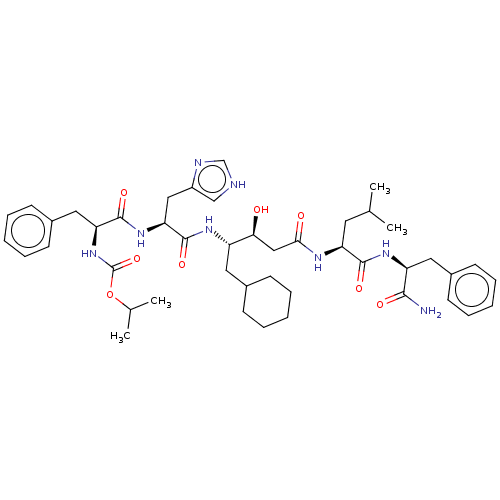
(CHEMBL3144415 | {1-[1-{3-[1-(1-Carbamoyl-2-phenyl-...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C45H64N8O8/c1-28(2)20-36(42(57)51-35(41(46)56)22-31-16-10-6-11-17-31)49-40(55)25-39(54)34(21-30-14-8-5-9-15-30)50-44(59)38(24-33-26-47-27-48-33)52-43(58)37(53-45(60)61-29(3)4)23-32-18-12-7-13-19-32/h6-7,10-13,16-19,26-30,34-39,54H,5,8-9,14-15,20-25H2,1-4H3,(H2,46,56)(H,47,48)(H,49,55)(H,50,59)(H,51,57)(H,52,58)(H,53,60) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50021129
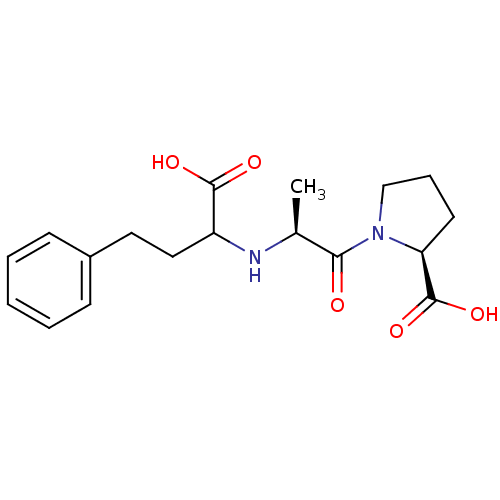
(1-[2-(1-Carboxy-3-phenyl-propylamino)-propionyl]-p...)Show SMILES C[C@H](NC(CCc1ccccc1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C18H24N2O5/c1-12(16(21)20-11-5-8-15(20)18(24)25)19-14(17(22)23)10-9-13-6-3-2-4-7-13/h2-4,6-7,12,14-15,19H,5,8-11H2,1H3,(H,22,23)(H,24,25)/t12-,14?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antihypertensive activity against human renin |
J Med Chem 24: 355-61 (1981)
BindingDB Entry DOI: 10.7270/Q2PC32XP |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023091
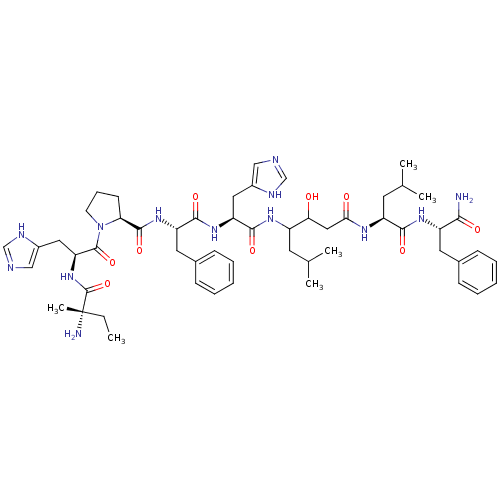
(CHEMBL269752 | Iva-His-Pro-Phe-His-Sta-Leu-Phe-NH2)Show SMILES CC[C@](C)(N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)NC(CC(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H77N13O9/c1-7-54(6,56)53(76)66-43(26-37-29-58-31-60-37)52(75)67-20-14-19-44(67)51(74)65-41(24-35-17-12-9-13-18-35)49(72)64-42(25-36-28-57-30-59-36)50(73)62-38(21-32(2)3)45(68)27-46(69)61-40(22-33(4)5)48(71)63-39(47(55)70)23-34-15-10-8-11-16-34/h8-13,15-18,28-33,38-45,68H,7,14,19-27,56H2,1-6H3,(H2,55,70)(H,57,59)(H,58,60)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)(H,66,76)/t38?,39-,40-,41-,42-,43-,44-,45?,54-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human kidney renin, fluorometric assay using a synthetic tetradecapeptide renin substrate at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antihypertensive activity against human renin |
J Med Chem 24: 355-61 (1981)
BindingDB Entry DOI: 10.7270/Q2PC32XP |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50212827
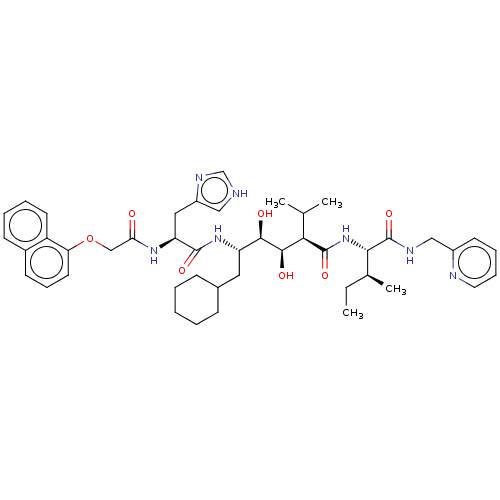
(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to renin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50281638
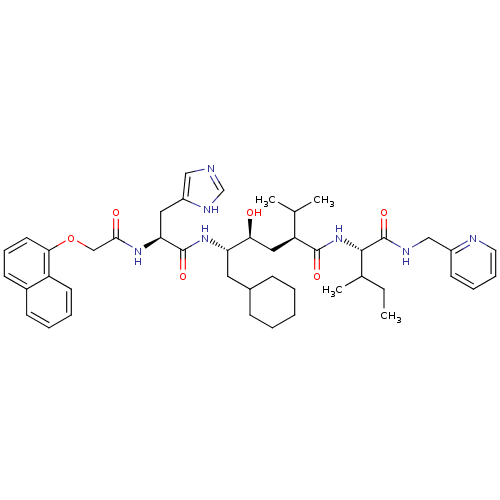
((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to renin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065395

(CHEMBL3401345)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)N2CC(CC2=O)c2cccc(Cl)c2)C(=N)N1 |r| Show InChI InChI=1S/C25H29ClN4O2/c1-16(2)25(3)13-23(32)30(24(27)28-25)14-17-6-4-9-21(10-17)29-15-19(12-22(29)31)18-7-5-8-20(26)11-18/h4-11,16,19H,12-15H2,1-3H3,(H2,27,28)/t19?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065426
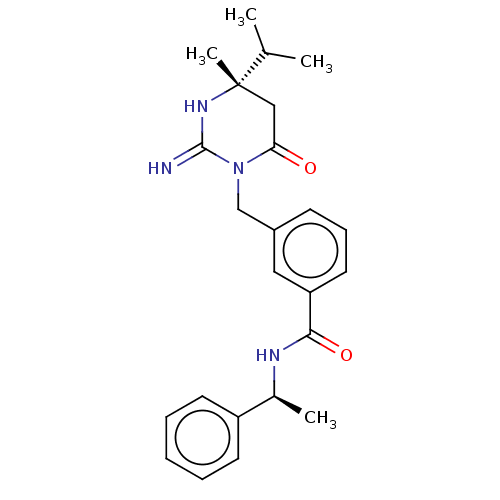
(CHEMBL3401348)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2cccc(c2)C(=O)N[C@@H](C)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H30N4O2/c1-16(2)24(4)14-21(29)28(23(25)27-24)15-18-9-8-12-20(13-18)22(30)26-17(3)19-10-6-5-7-11-19/h5-13,16-17H,14-15H2,1-4H3,(H2,25,27)(H,26,30)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50333945
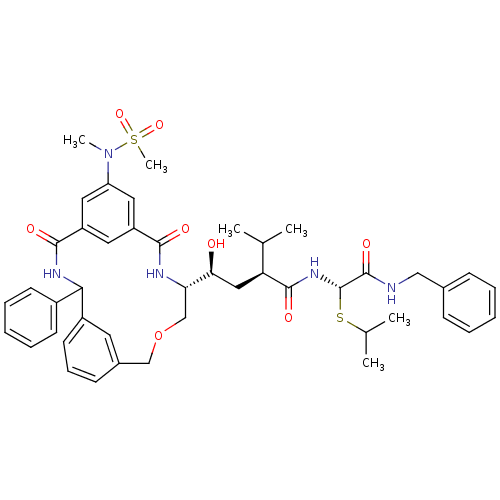
((S)-N-((S)-Benzylcarbamoyl-isopropylsulfanyl-methy...)Show SMILES CC(C)S[C@H](NC(=O)[C@@H](C[C@H](O)[C@@H]1COCc2cccc(c2)C(NC(=O)c2cc(cc(c2)C(=O)N1)N(C)S(C)(=O)=O)c1ccccc1)C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C45H55N5O8S2/c1-28(2)37(43(54)49-45(59-29(3)4)44(55)46-25-30-14-9-7-10-15-30)24-39(51)38-27-58-26-31-16-13-19-33(20-31)40(32-17-11-8-12-18-32)48-42(53)35-21-34(41(52)47-38)22-36(23-35)50(5)60(6,56)57/h7-23,28-29,37-40,45,51H,24-27H2,1-6H3,(H,46,55)(H,47,52)(H,48,53)(H,49,54)/t37-,38-,39-,40?,45-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of renin |
Bioorg Med Chem Lett 21: 358-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.140
BindingDB Entry DOI: 10.7270/Q2HM58RJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025932

(CHEMBL3144406 | Nitrate salt of {1-[1-(1-{2-[1-(1-...)Show SMILES O[N+]([O-])=O.CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)NCc1cccc(CN=C(N)N)c1 |r,wU:12.11,44.45,38.39,wD:22.22,8.6,(-1.54,,;;.77,1.33,;.77,-1.33,;18.37,2.44,;19.8,3.03,;20,4.56,;21.02,2.09,;20.81,.56,;22.03,-.38,;23.46,.21,;23.66,1.74,;24.68,-.73,;24.47,-2.25,;23.05,-2.84,;22.69,-4.34,;21.15,-4.46,;20.57,-3.03,;21.74,-2.03,;26.1,-.14,;27.32,-1.08,;27.12,-2.61,;28.75,-.49,;28.95,1.03,;30.37,1.62,;30.58,3.15,;32,3.73,;33.22,2.8,;33.02,1.27,;31.59,.68,;29.97,-1.43,;29.76,-2.96,;30.98,-3.9,;28.34,-3.55,;28.14,-5.07,;27.93,-6.6,;26.61,-4.87,;29.66,-5.28,;19.39,-.02,;19.19,-1.55,;18.17,.92,;16.75,.33,;16.54,-1.2,;15.53,1.27,;14.1,.68,;13.9,-.84,;12.48,-1.43,;12.27,-2.96,;11.25,-.49,;12.88,1.62,;13.09,3.15,;11.46,1.03,;10.24,1.97,;8.81,1.39,;8.61,-.14,;7.19,-.73,;5.97,.21,;6.17,1.74,;4.95,2.68,;5.15,4.2,;6.58,4.79,;6.78,6.32,;7.8,3.85,;7.59,2.33,)| Show InChI InChI=1S/C43H64N10O7/c1-26(2)16-32(36(54)21-37(55)50-33(17-27(3)4)38(56)47-22-29-14-11-15-30(18-29)23-48-41(44)45)51-40(58)35(20-31-24-46-25-49-31)52-39(57)34(19-28-12-9-8-10-13-28)53-42(59)60-43(5,6)7/h8-15,18,24-27,32-36,54H,16-17,19-23H2,1-7H3,(H,46,49)(H,47,56)(H,50,55)(H,51,58)(H,52,57)(H,53,59)(H4,44,45,48) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human plasma renin |
J Med Chem 28: 1755-6 (1986)
BindingDB Entry DOI: 10.7270/Q2P26X56 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405190

(CHEMBL2028991)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H80N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h6-7,10-13,17-20,31-37,42-49,70H,5,8-9,14-16,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023050

(CHEMBL40928 | {1-[1-(1-{2-[1-(1-Carbamoyl-2-phenyl...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H64N6O8/c1-29(2)23-34(39(53)28-40(54)48-36(24-30(3)4)42(56)50-35(41(47)55)25-31-17-11-8-12-18-31)49-43(57)37(26-32-19-13-9-14-20-32)51-44(58)38(27-33-21-15-10-16-22-33)52-45(59)60-46(5,6)7/h8-22,29-30,34-39,53H,23-28H2,1-7H3,(H2,47,55)(H,48,54)(H,49,57)(H,50,56)(H,51,58)(H,52,59)/t34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human kidney renin |
J Med Chem 28: 1755-6 (1986)
BindingDB Entry DOI: 10.7270/Q2P26X56 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065424
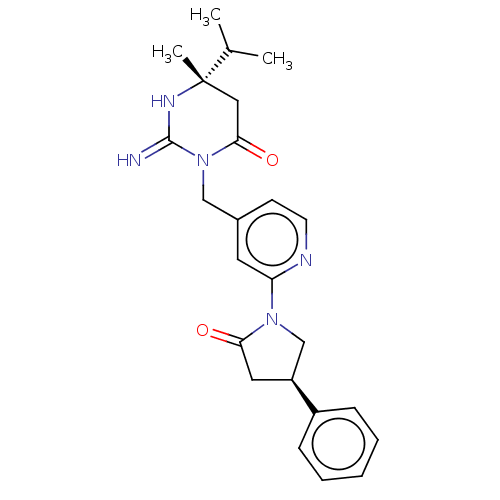
(CHEMBL3401346)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2C[C@H](CC2=O)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H29N5O2/c1-16(2)24(3)13-22(31)29(23(25)27-24)14-17-9-10-26-20(11-17)28-15-19(12-21(28)30)18-7-5-4-6-8-18/h4-11,16,19H,12-15H2,1-3H3,(H2,25,27)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023096

(Boc-Phe-His-Sta-Leu-(+)-alpha-phenylthylamide | CH...)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)NC(C)c1ccccc1 Show InChI InChI=1S/C42H61N7O7/c1-26(2)19-32(36(50)23-37(51)46-33(20-27(3)4)38(52)45-28(5)30-17-13-10-14-18-30)47-40(54)35(22-31-24-43-25-44-31)48-39(53)34(21-29-15-11-9-12-16-29)49-41(55)56-42(6,7)8/h9-18,24-28,32-36,50H,19-23H2,1-8H3,(H,43,44)(H,45,52)(H,46,51)(H,47,54)(H,48,53)(H,49,55)/t28?,32?,33-,34-,35-,36?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human kidney renin, radioimmunoassay using a synthetic tetradecapeptide renin substrate at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405191
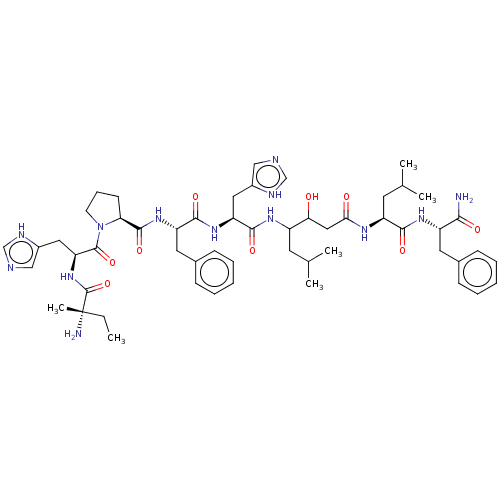
(CHEMBL269752)Show SMILES CC[C@](C)(N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)NC(CC(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H77N13O9/c1-7-54(6,56)53(76)66-43(26-37-29-58-31-60-37)52(75)67-20-14-19-44(67)51(74)65-41(24-35-17-12-9-13-18-35)49(72)64-42(25-36-28-57-30-59-36)50(73)62-38(21-32(2)3)45(68)27-46(69)61-40(22-33(4)5)48(71)63-39(47(55)70)23-34-15-10-8-11-16-34/h8-13,15-18,28-33,38-45,68H,7,14,19-27,56H2,1-6H3,(H2,55,70)(H,57,59)(H,58,60)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)(H,66,76)/t38?,39-,40-,41-,42-,43-,44-,45?,54-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of rabbit plasma renin. |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023054

(CHEMBL291091 | {1-[1-(1-{2-[1-(1-Carbamoyl-2-pheny...)Show SMILES CC(C)C[C@H](NC(=O)C(F)(F)C(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C46H60F2N6O8/c1-28(2)23-33(38(55)46(47,48)43(60)53-35(24-29(3)4)40(57)51-34(39(49)56)25-30-17-11-8-12-18-30)50-41(58)36(26-31-19-13-9-14-20-31)52-42(59)37(27-32-21-15-10-16-22-32)54-44(61)62-45(5,6)7/h8-22,28-29,33-37H,23-27H2,1-7H3,(H2,49,56)(H,50,58)(H,51,57)(H,52,59)(H,53,60)(H,54,61)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human kidney renin |
J Med Chem 30: 1617-22 (1987)
BindingDB Entry DOI: 10.7270/Q2RB73KD |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50333945

((S)-N-((S)-Benzylcarbamoyl-isopropylsulfanyl-methy...)Show SMILES CC(C)S[C@H](NC(=O)[C@@H](C[C@H](O)[C@@H]1COCc2cccc(c2)C(NC(=O)c2cc(cc(c2)C(=O)N1)N(C)S(C)(=O)=O)c1ccccc1)C(C)C)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C45H55N5O8S2/c1-28(2)37(43(54)49-45(59-29(3)4)44(55)46-25-30-14-9-7-10-15-30)24-39(51)38-27-58-26-31-16-13-19-33(20-31)40(32-17-11-8-12-18-32)48-42(53)35-21-34(41(52)47-38)22-36(23-35)50(5)60(6,56)57/h7-23,28-29,37-40,45,51H,24-27H2,1-6H3,(H,46,55)(H,47,52)(H,48,53)(H,49,54)/t37-,38-,39-,40?,45-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
Bioorg Med Chem Lett 21: 358-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.140
BindingDB Entry DOI: 10.7270/Q2HM58RJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025946
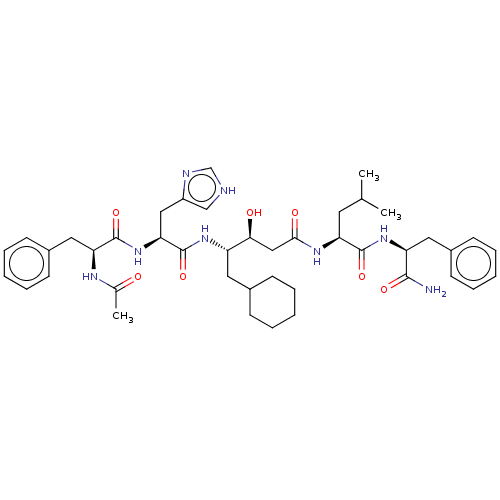
(4-[2-(2-Acetylamino-3-phenyl-propionylamino)-3-(1H...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C43H60N8O7/c1-27(2)19-35(41(56)50-34(40(44)55)21-30-15-9-5-10-16-30)48-39(54)24-38(53)33(20-29-13-7-4-8-14-29)49-43(58)37(23-32-25-45-26-46-32)51-42(57)36(47-28(3)52)22-31-17-11-6-12-18-31/h5-6,9-12,15-18,25-27,29,33-38,53H,4,7-8,13-14,19-24H2,1-3H3,(H2,44,55)(H,45,46)(H,47,52)(H,48,54)(H,49,58)(H,50,56)(H,51,57) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human kidney renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022507
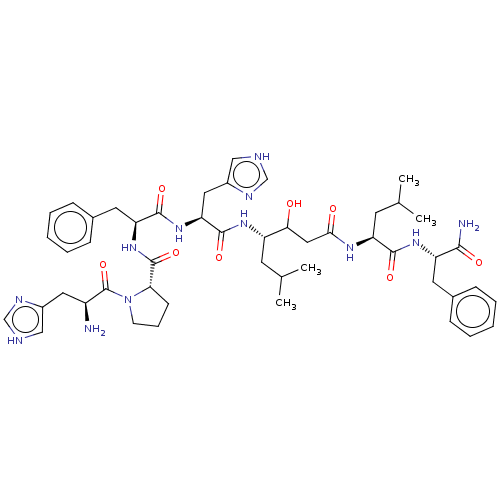
(CHEMBL3349407 | H2N-His-Pro-Phe-His-Sta-Leu-PheOMe)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1c[nH]cn1)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H68N12O8/c1-29(2)18-36(42(62)24-43(63)56-38(19-30(3)4)45(65)58-37(44(51)64)20-31-12-7-5-8-13-31)57-47(67)40(23-34-26-53-28-55-34)59-46(66)39(21-32-14-9-6-10-15-32)60-48(68)41-16-11-17-61(41)49(69)35(50)22-33-25-52-27-54-33/h5-10,12-15,25-30,35-42,62H,11,16-24,50H2,1-4H3,(H2,51,64)(H,52,54)(H,53,55)(H,56,63)(H,57,67)(H,58,65)(H,59,66)(H,60,68)/t35-,36?,37-,38-,39-,40-,41-,42?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin
Curated by ChEMBL
| Assay Description
Inhibition of human renin at pH 7.4 |
J Med Chem 31: 625-9 (1988)
BindingDB Entry DOI: 10.7270/Q2B85742 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405191

(CHEMBL269752)Show SMILES CC[C@](C)(N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)NC(CC(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H77N13O9/c1-7-54(6,56)53(76)66-43(26-37-29-58-31-60-37)52(75)67-20-14-19-44(67)51(74)65-41(24-35-17-12-9-13-18-35)49(72)64-42(25-36-28-57-30-59-36)50(73)62-38(21-32(2)3)45(68)27-46(69)61-40(22-33(4)5)48(71)63-39(47(55)70)23-34-15-10-8-11-16-34/h8-13,15-18,28-33,38-45,68H,7,14,19-27,56H2,1-6H3,(H2,55,70)(H,57,59)(H,58,60)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)(H,66,76)/t38?,39-,40-,41-,42-,43-,44-,45?,54-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human kidney renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022520
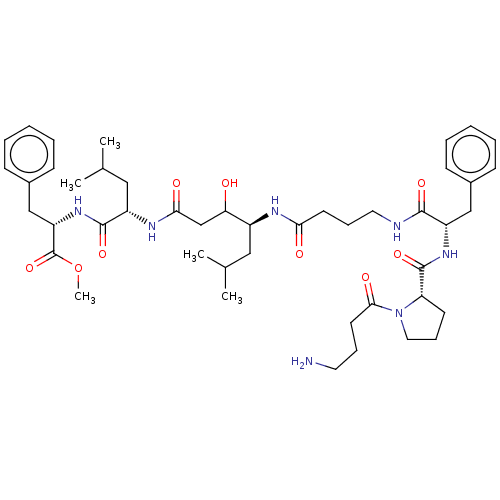
(CHEMBL3349395 | H2N-Abu-Pro-Phe-Abu-Sta-Leu-PheOMe)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)CC(O)[C@H](CC(C)C)NC(=O)CCCNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)CCCN Show InChI InChI=1S/C46H69N7O9/c1-30(2)25-34(39(54)29-41(56)50-35(26-31(3)4)44(59)52-37(46(61)62-5)28-33-17-10-7-11-18-33)49-40(55)20-13-23-48-43(58)36(27-32-15-8-6-9-16-32)51-45(60)38-19-14-24-53(38)42(57)21-12-22-47/h6-11,15-18,30-31,34-39,54H,12-14,19-29,47H2,1-5H3,(H,48,58)(H,49,55)(H,50,56)(H,51,60)(H,52,59)/t34?,35-,36-,37-,38-,39?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin
Curated by ChEMBL
| Assay Description
Inhibition of human renin at pH 7.4 |
J Med Chem 31: 625-9 (1988)
BindingDB Entry DOI: 10.7270/Q2B85742 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405191

(CHEMBL269752)Show SMILES CC[C@](C)(N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)NC(CC(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H77N13O9/c1-7-54(6,56)53(76)66-43(26-37-29-58-31-60-37)52(75)67-20-14-19-44(67)51(74)65-41(24-35-17-12-9-13-18-35)49(72)64-42(25-36-28-57-30-59-36)50(73)62-38(21-32(2)3)45(68)27-46(69)61-40(22-33(4)5)48(71)63-39(47(55)70)23-34-15-10-8-11-16-34/h8-13,15-18,28-33,38-45,68H,7,14,19-27,56H2,1-6H3,(H2,55,70)(H,57,59)(H,58,60)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)(H,66,76)/t38?,39-,40-,41-,42-,43-,44-,45?,54-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023088

(Boc-Phe-His-Sta-Leu-p-methoxybenzylamide | CHEMBL5...)Show SMILES COc1ccc(CNC(=O)[C@H](CC(C)C)NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C42H61N7O8/c1-26(2)18-32(36(50)22-37(51)46-33(19-27(3)4)38(52)44-23-29-14-16-31(56-8)17-15-29)47-40(54)35(21-30-24-43-25-45-30)48-39(53)34(20-28-12-10-9-11-13-28)49-41(55)57-42(5,6)7/h9-17,24-27,32-36,50H,18-23H2,1-8H3,(H,43,45)(H,44,52)(H,46,51)(H,47,54)(H,48,53)(H,49,55)/t32?,33-,34-,35-,36?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50084688
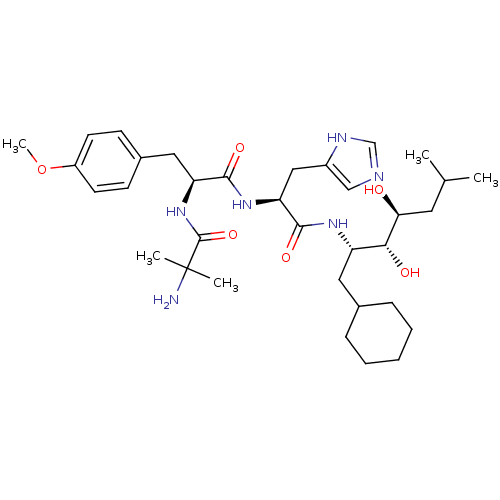
(2-Amino-N-[1-[1-(1-cyclohexylmethyl-2,3-dihydroxy-...)Show SMILES COc1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](CC2CCCCC2)[C@@H](O)[C@@H](O)CC(C)C)cc1 Show InChI InChI=1S/C34H54N6O6/c1-21(2)15-29(41)30(42)26(16-22-9-7-6-8-10-22)38-32(44)28(18-24-19-36-20-37-24)39-31(43)27(40-33(45)34(3,4)35)17-23-11-13-25(46-5)14-12-23/h11-14,19-22,26-30,41-42H,6-10,15-18,35H2,1-5H3,(H,36,37)(H,38,44)(H,39,43)(H,40,45)/t26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland
Curated by ChEMBL
| Assay Description
Binding affinity against aspartic protease renin. |
J Med Chem 43: 305-41 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2JD4XH4 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50025948

(5-Cyclohexyl-3-hydroxy-4-[3-(1H-imidazol-4-yl)-2-(...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H55N7O7/c1-26(2)18-33(39(52)47-32(38(41)51)20-28-14-8-4-9-15-28)44-36(49)22-35(48)31(19-27-12-6-3-7-13-27)46-40(53)34(21-29-23-42-25-43-29)45-37(50)24-54-30-16-10-5-11-17-30/h4-5,8-11,14-17,23,25-27,31-35,48H,3,6-7,12-13,18-22,24H2,1-2H3,(H2,41,51)(H,42,43)(H,44,49)(H,45,50)(H,46,53)(H,47,52) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50065425
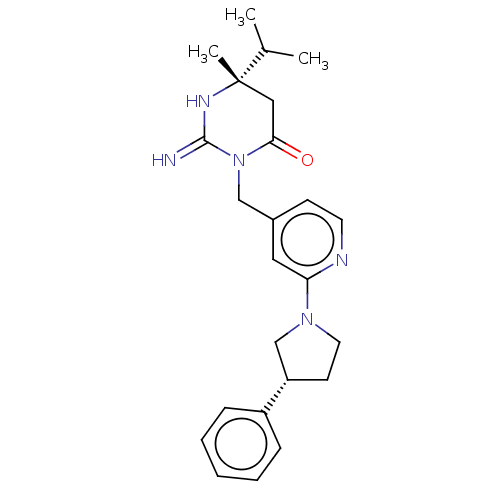
(CHEMBL3401347)Show SMILES CC(C)[C@]1(C)CC(=O)N(Cc2ccnc(c2)N2CC[C@@H](C2)c2ccccc2)C(=N)N1 |r| Show InChI InChI=1S/C24H31N5O/c1-17(2)24(3)14-22(30)29(23(25)27-24)15-18-9-11-26-21(13-18)28-12-10-20(16-28)19-7-5-4-6-8-19/h4-9,11,13,17,20H,10,12,14-16H2,1-3H3,(H2,25,27)/t20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of renin (unknown origin) |
Bioorg Med Chem Lett 25: 1592-6 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.003
BindingDB Entry DOI: 10.7270/Q2X63PMJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022519
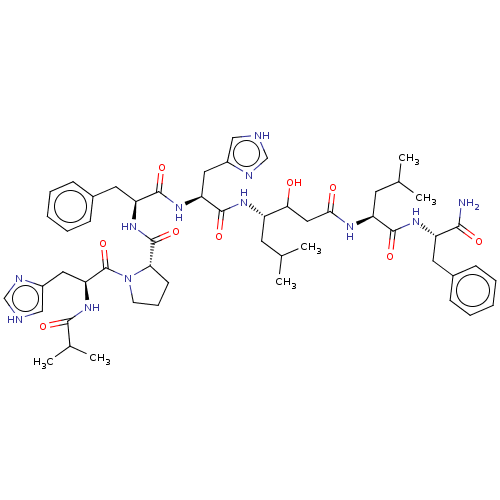
(CHEMBL3349393 | Ibu-His-Pro-Phe-His-Sta-Leu-PheNH2)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)C(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H74N12O9/c1-31(2)20-38(45(66)26-46(67)59-40(21-32(3)4)49(70)61-39(47(54)68)22-34-14-9-7-10-15-34)60-51(72)42(24-36-27-55-29-57-36)62-50(71)41(23-35-16-11-8-12-17-35)63-52(73)44-18-13-19-65(44)53(74)43(64-48(69)33(5)6)25-37-28-56-30-58-37/h7-12,14-17,27-33,38-45,66H,13,18-26H2,1-6H3,(H2,54,68)(H,55,57)(H,56,58)(H,59,67)(H,60,72)(H,61,70)(H,62,71)(H,63,73)(H,64,69)/t38?,39-,40-,41-,42-,43?,44-,45?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Wisconsin
Curated by ChEMBL
| Assay Description
Inhibition of human renin at pH 7.4 |
J Med Chem 31: 625-9 (1988)
BindingDB Entry DOI: 10.7270/Q2B85742 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50023091

(CHEMBL269752 | Iva-His-Pro-Phe-His-Sta-Leu-Phe-NH2)Show SMILES CC[C@](C)(N)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)NC(CC(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H77N13O9/c1-7-54(6,56)53(76)66-43(26-37-29-58-31-60-37)52(75)67-20-14-19-44(67)51(74)65-41(24-35-17-12-9-13-18-35)49(72)64-42(25-36-28-57-30-59-36)50(73)62-38(21-32(2)3)45(68)27-46(69)61-40(22-33(4)5)48(71)63-39(47(55)70)23-34-15-10-8-11-16-34/h8-13,15-18,28-33,38-45,68H,7,14,19-27,56H2,1-6H3,(H2,55,70)(H,57,59)(H,58,60)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)(H,66,76)/t38?,39-,40-,41-,42-,43-,44-,45?,54-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human kidney renin, radioimmunoassay using the natural substrate partially pure angiotensinogen at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50016033

(CHEMBL3085574 | {1-[1-{1-[2-(1-Benzylcarbamoyl-3-m...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)NCc1ccccc1 Show InChI InChI=1S/C41H59N7O7/c1-26(2)18-31(35(49)22-36(50)45-32(19-27(3)4)37(51)43-23-29-16-12-9-13-17-29)46-39(53)34(21-30-24-42-25-44-30)47-38(52)33(20-28-14-10-8-11-15-28)48-40(54)55-41(5,6)7/h8-17,24-27,31-35,49H,18-23H2,1-7H3,(H,42,44)(H,43,51)(H,45,50)(H,46,53)(H,47,52)(H,48,54)/t31-,32-,33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of purified human kidney renin, radioimmunoassay using the natural substrate partially pure angiotensinogen at 10e-9 M concentration |
J Med Chem 30: 1853-7 (1987)
BindingDB Entry DOI: 10.7270/Q2C24VDZ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405192
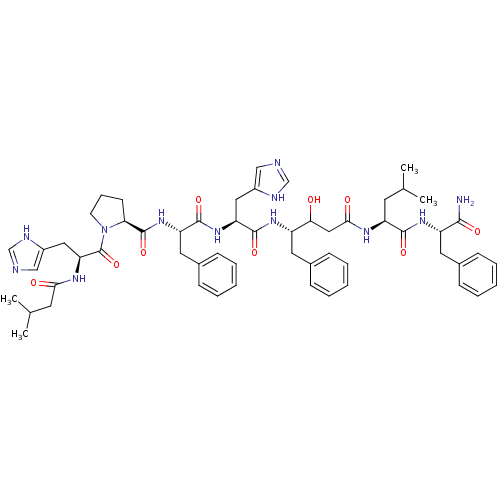
(CHEMBL2028990)Show SMILES CC(C)C[C@H](NC(=O)CC(O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C57H74N12O9/c1-35(2)23-44(53(74)66-43(52(58)73)26-38-17-10-6-11-18-38)63-51(72)30-49(70)42(25-37-15-8-5-9-16-37)65-55(76)46(28-40-31-59-33-61-40)67-54(75)45(27-39-19-12-7-13-20-39)68-56(77)48-21-14-22-69(48)57(78)47(29-41-32-60-34-62-41)64-50(71)24-36(3)4/h5-13,15-20,31-36,42-49,70H,14,21-30H2,1-4H3,(H2,58,73)(H,59,61)(H,60,62)(H,63,72)(H,64,71)(H,65,76)(H,66,74)(H,67,75)(H,68,77)/t42-,43-,44-,45-,46-,47-,48-,49?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405191

(CHEMBL269752)Show SMILES CC[C@](C)(N)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)NC(CC(C)C)C(O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H77N13O9/c1-7-54(6,56)53(76)66-43(26-37-29-58-31-60-37)52(75)67-20-14-19-44(67)51(74)65-41(24-35-17-12-9-13-18-35)49(72)64-42(25-36-28-57-30-59-36)50(73)62-38(21-32(2)3)45(68)27-46(69)61-40(22-33(4)5)48(71)63-39(47(55)70)23-34-15-10-8-11-16-34/h8-13,15-18,28-33,38-45,68H,7,14,19-27,56H2,1-6H3,(H2,55,70)(H,57,59)(H,58,60)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)(H,66,76)/t38?,39-,40-,41-,42-,43-,44-,45?,54-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human kidney renin |
J Med Chem 28: 1779-90 (1986)
BindingDB Entry DOI: 10.7270/Q2FJ2HC2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data