 Found 4023 hits of ic50 for UniProtKB: P05177
Found 4023 hits of ic50 for UniProtKB: P05177 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02071
BindingDB Entry DOI: 10.7270/Q2833X3S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50609928
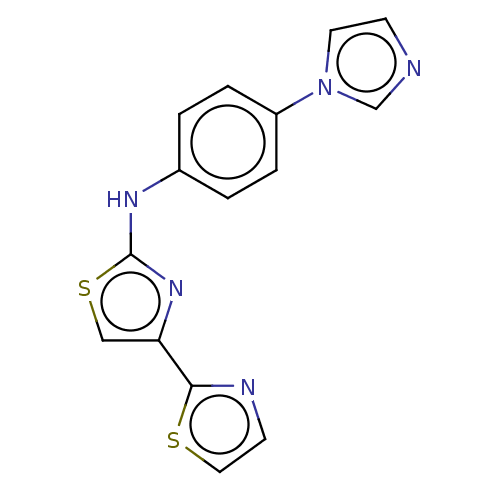
(CHEMBL5279681) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.125 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50507479

(CHEMBL4447260)Show InChI InChI=1S/C18H18N2O2S/c1-21-16-11-15(4-5-18-3-2-10-23-18)12-17(13-16)22-9-8-20-7-6-19-14-20/h2-7,10-14H,8-9H2,1H3/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1A2 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... |
ACS Med Chem Lett 9: 1247-1252 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00409
BindingDB Entry DOI: 10.7270/Q2V1284K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate incubated for 10 mins in presence of NADPH by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01559
BindingDB Entry DOI: 10.7270/Q27P9361 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50325213

(2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...)Show SMILES CC(C)(C)c1nnc(s1)-c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H20BrCl2N7S/c1-24(2,3)23-31-30-22(35-23)20-17(11-33-13-28-12-29-33)21(14-4-6-15(25)7-5-14)34(32-20)19-9-8-16(26)10-18(19)27/h4-10,12-13H,11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes after 30 mins |
Bioorg Med Chem 18: 6377-88 (2010)
Article DOI: 10.1016/j.bmc.2010.07.013
BindingDB Entry DOI: 10.7270/Q2XG9RBT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 assessed as reduction in 7-ethoxyresorufin O-deethylation activity by fluorescence based EROD assay |
J Med Chem 58: 3534-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00265
BindingDB Entry DOI: 10.7270/Q2XP76NJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50420191
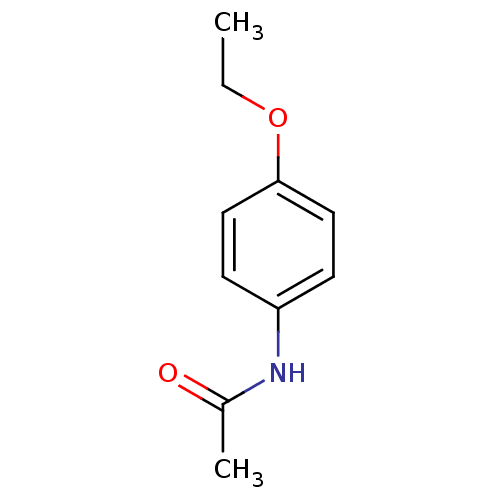
(ACETPHENETIDIN | Acetophenetidin | PHENACETIN)Show InChI InChI=1S/C10H13NO2/c1-3-13-10-6-4-9(5-7-10)11-8(2)12/h4-7H,3H2,1-2H3,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114512
BindingDB Entry DOI: 10.7270/Q2TH8RR7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval,
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 expressed in Escherichia coli DH5alpha coexpressing human NADPH P450 reductase in using 7-ethoxyresorufin as substrate in ... |
Eur J Med Chem 135: 296-306 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.042
BindingDB Entry DOI: 10.7270/Q26Q20QW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 expressed in Escherichia coli membranes assessed as reduction in 7-ethoxyresorufin O-deethylation activity |
J Med Chem 58: 3534-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00265
BindingDB Entry DOI: 10.7270/Q2XP76NJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem 15: 5047-60 (2007)
Article DOI: 10.1016/j.bmc.2007.05.046
BindingDB Entry DOI: 10.7270/Q20R9Q7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1A2 expressed in baculovirus infected insect cells using 7-ethoxyresorufin as substrate preincubated for 5 mins fo... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.112028
BindingDB Entry DOI: 10.7270/Q26M3BG7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50214608
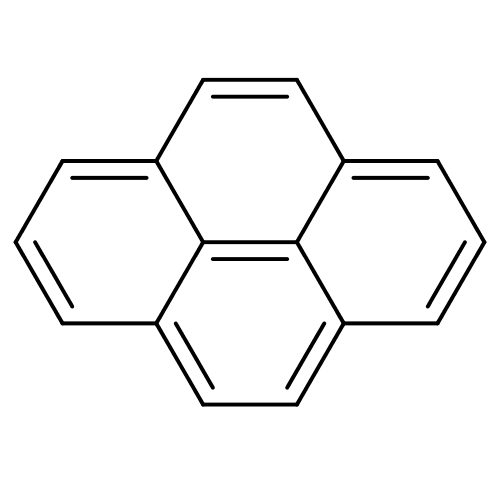
(CHEMBL279564 | pyrene)Show InChI InChI=1S/C16H10/c1-3-11-7-9-13-5-2-6-14-10-8-12(4-1)15(11)16(13)14/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem 15: 5047-60 (2007)
Article DOI: 10.1016/j.bmc.2007.05.046
BindingDB Entry DOI: 10.7270/Q20R9Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50136037
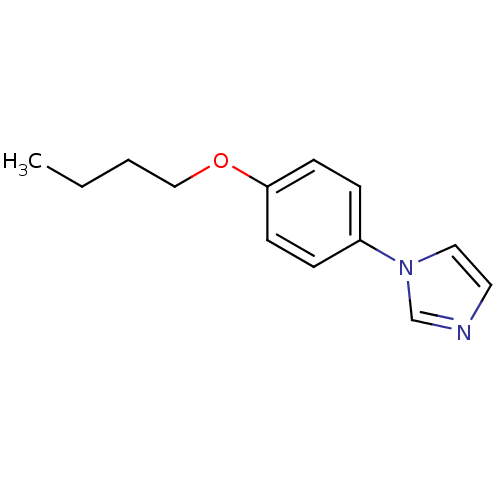
(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cytochrome P450 1A2. |
J Med Chem 46: 5416-27 (2003)
Article DOI: 10.1021/jm020557k
BindingDB Entry DOI: 10.7270/Q2QF8S96 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50136037

(1-(4-Butoxy-phenyl)-1H-imidazole | CHEMBL112532)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-16-13-6-4-12(5-7-13)15-9-8-14-11-15/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of cytochrome P450 1A2 |
Bioorg Med Chem Lett 14: 333-6 (2003)
BindingDB Entry DOI: 10.7270/Q2JD4W74 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00468
BindingDB Entry DOI: 10.7270/Q29027TT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50259240
((5-(1-Benzyl-1H-indazol-3-yl)-1,2,4-oxadiazol-3-yl...)Show InChI InChI=1S/C17H15N5O/c18-10-15-19-17(23-21-15)16-13-8-4-5-9-14(13)22(20-16)11-12-6-2-1-3-7-12/h1-9H,10-11,18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 |
J Med Chem 52: 2694-707 (2009)
Article DOI: 10.1021/jm801180p
BindingDB Entry DOI: 10.7270/Q2GT5N3B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50335818
(6-(3-Hydroxyphenyl)-1-(pyridin-3-yl)-2-naphthol | ...)Show InChI InChI=1S/C21H15NO2/c23-18-5-1-3-14(12-18)15-6-8-19-16(11-15)7-9-20(24)21(19)17-4-2-10-22-13-17/h1-13,23-24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 |
J Med Chem 54: 534-47 (2011)
Article DOI: 10.1021/jm1009082
BindingDB Entry DOI: 10.7270/Q2WH2Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM143355
(US9682953, 20.A-1)Show SMILES CCOC(=O)Cn1nc2C(=O)N(C(c2c1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C Show InChI InChI=1S/C25H22F3N3O2/c26-25(27,28)20-8-11-23(30-16-20)33-22-5-1-3-18(14-22)13-17-6-9-21(10-7-17)31-24(32)19-4-2-12-29-15-19/h1-5,8,11-16,21H,6-7,9-10H2,(H,31,32)/b17-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50131050

(1-Methoxy-8,13-dihydro-7H-indolo[2',3':3,4]pyrido[...)Show InChI InChI=1S/C19H15N3O2/c1-24-15-8-4-6-13-16(15)21-18-17-12(9-10-22(18)19(13)23)11-5-2-3-7-14(11)20-17/h2-8,20H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Institute of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Cytochrome P450 1A2 enzyme in bacterial membrane expressing human P450s |
Bioorg Med Chem Lett 13: 2535-8 (2003)
BindingDB Entry DOI: 10.7270/Q2805341 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50108048
((E)-2-(3,5-dimethoxystyryl)thiophene | 2-[(E)-2-(3...)Show InChI InChI=1S/C14H14O2S/c1-15-12-8-11(9-13(10-12)16-2)5-6-14-4-3-7-17-14/h3-10H,1-2H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1A2 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... |
ACS Med Chem Lett 9: 1247-1252 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00409
BindingDB Entry DOI: 10.7270/Q2V1284K |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50159640

(2-(4-Chloro-phenyl)-benzo[h]chromen-4-one | CHEMBL...)Show InChI InChI=1S/C19H11ClO2/c20-14-8-5-13(6-9-14)18-11-17(21)16-10-7-12-3-1-2-4-15(12)19(16)22-18/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate in presence of glucose-6-phosphate, glucose-6-phosphate dehydrogenase and... |
Eur J Med Chem 187: (2020)
Article DOI: 10.1016/j.ejmech.2019.111938
BindingDB Entry DOI: 10.7270/Q2SX6HMM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50108048

((E)-2-(3,5-dimethoxystyryl)thiophene | 2-[(E)-2-(3...)Show InChI InChI=1S/C14H14O2S/c1-15-12-8-11(9-13(10-12)16-2)5-6-14-4-3-7-17-14/h3-10H,1-2H3/b6-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1A2 |
J Med Chem 45: 160-4 (2001)
BindingDB Entry DOI: 10.7270/Q2668CGD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50134367

(2-[(Z)-2-(3,5-Dimethoxy-phenyl)-vinyl]-thiophene |...)Show InChI InChI=1S/C14H14O2S/c1-15-12-8-11(9-13(10-12)16-2)5-6-14-4-3-7-17-14/h3-10H,1-2H3/b6-5- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
Bioorg Med Chem 15: 5047-60 (2007)
Article DOI: 10.1016/j.bmc.2007.05.046
BindingDB Entry DOI: 10.7270/Q20R9Q7F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
De Montfort University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in supersomes (unknown origin) |
Eur J Med Chem 129: 159-174 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.016
BindingDB Entry DOI: 10.7270/Q26T0PX4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate incubated for 5 mins followed by NADPH addition and measured after 20 mi... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112465
BindingDB Entry DOI: 10.7270/Q23X8BCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50159609
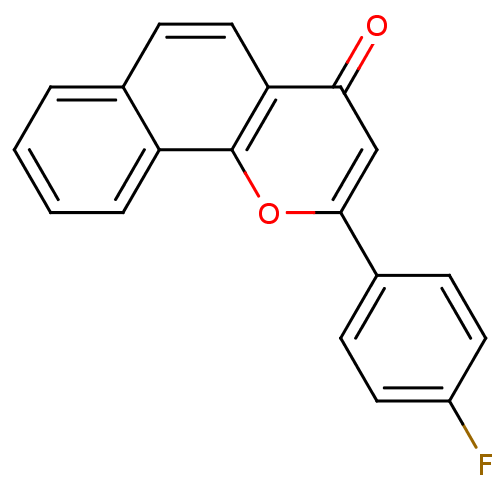
(2-(4-Fluoro-phenyl)-benzo[h]chromen-4-one | CHEMBL...)Show InChI InChI=1S/C19H11FO2/c20-14-8-5-13(6-9-14)18-11-17(21)16-10-7-12-3-1-2-4-15(12)19(16)22-18/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate in presence of glucose-6-phosphate, glucose-6-phosphate dehydrogenase and... |
Eur J Med Chem 187: (2020)
Article DOI: 10.1016/j.ejmech.2019.111938
BindingDB Entry DOI: 10.7270/Q2SX6HMM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060418
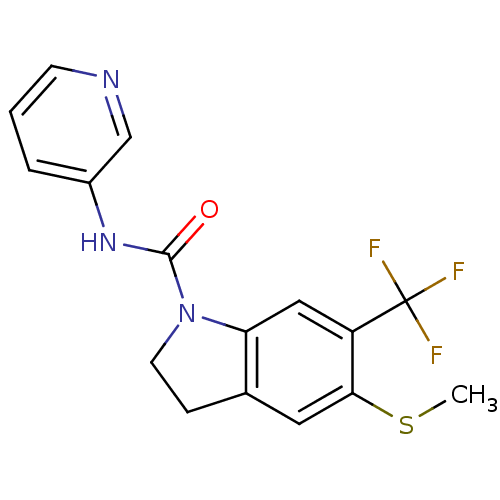
(5-Methylsulfanyl-6-trifluoromethyl-2,3-dihydro-ind...)Show InChI InChI=1S/C16H14F3N3OS/c1-24-14-7-10-4-6-22(13(10)8-12(14)16(17,18)19)15(23)21-11-3-2-5-20-9-11/h2-3,5,7-9H,4,6H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of heterologously expressed human Cytochrome P450 1A2 at 500 uM |
J Med Chem 40: 3494-6 (1997)
Article DOI: 10.1021/jm970424c
BindingDB Entry DOI: 10.7270/Q2PG1QTG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50060418

(5-Methylsulfanyl-6-trifluoromethyl-2,3-dihydro-ind...)Show InChI InChI=1S/C16H14F3N3OS/c1-24-14-7-10-4-6-22(13(10)8-12(14)16(17,18)19)15(23)21-11-3-2-5-20-9-11/h2-3,5,7-9H,4,6H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of heterologously expressed human cytochrome P450 1A2 |
J Med Chem 43: 1123-34 (2000)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23X85V5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM143362
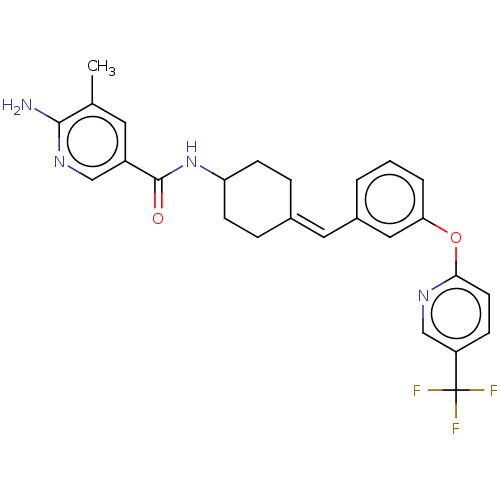
(US9682953, 20.A-5)Show SMILES Cc1cc(cnc1N)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(11.34,-.39,;10,.38,;8.67,-.38,;7.34,.38,;7.34,1.93,;8.67,2.69,;10,1.93,;11.34,2.69,;6,-.38,;6,-1.93,;4.67,.38,;3.33,-.38,;3.33,-1.93,;2,-2.69,;.67,-1.93,;.67,-.38,;2,.38,;-.67,-2.69,;-2,-1.93,;-2,-.38,;-3.33,.38,;-4.67,-.38,;-4.67,-1.93,;-6,-2.69,;-7.34,-1.93,;-7.34,-.38,;-8.67,.38,;-10,-.38,;-10,-1.93,;-8.67,-2.69,;-11.34,.38,;-12.67,-.39,;-11.34,1.93,;-11.34,-1.16,;-3.33,-2.69,)| Show InChI InChI=1S/C26H25F3N4O2/c1-16-11-19(14-32-24(16)30)25(34)33-21-8-5-17(6-9-21)12-18-3-2-4-22(13-18)35-23-10-7-20(15-31-23)26(27,28)29/h2-4,7,10-15,21H,5-6,8-9H2,1H3,(H2,30,32)(H,33,34)/b17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >13 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM143373

(US9682953, 20.A-10 | US9682953, 20.A-9)Show SMILES Nc1ncc(cn1)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(11.34,2.69,;10,1.93,;10,.38,;8.67,-.38,;7.34,.38,;7.34,1.93,;8.67,2.69,;6,-.38,;6,-1.93,;4.67,.38,;3.33,-.38,;3.33,-1.93,;2,-2.69,;.67,-1.93,;.67,-.38,;2,.38,;-.67,-2.69,;-2,-1.93,;-2,-.38,;-3.33,.38,;-4.67,-.38,;-4.67,-1.93,;-6,-2.69,;-7.34,-1.93,;-7.34,-.38,;-8.67,.38,;-10,-.38,;-10,-1.93,;-8.67,-2.69,;-11.34,.38,;-12.67,-.39,;-11.34,1.93,;-11.34,-1.16,;-3.33,-2.69,)| Show InChI InChI=1S/C24H22F3N5O2/c25-24(26,27)18-6-9-21(29-14-18)34-20-3-1-2-16(11-20)10-15-4-7-19(8-5-15)32-22(33)17-12-30-23(28)31-13-17/h1-3,6,9-14,19H,4-5,7-8H2,(H,32,33)(H2,28,30,31)/b15-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >13 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM143359
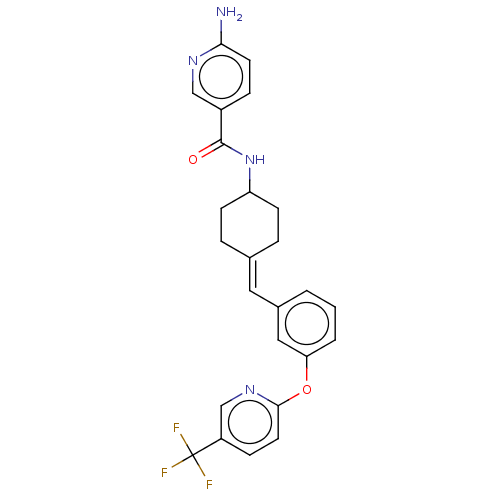
(US9682953, 20.A-3)Show SMILES Nc1ccc(cn1)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(12.65,-1.17,;11.31,-1.94,;11.31,-3.48,;9.98,-4.25,;8.65,-3.48,;8.65,-1.94,;9.98,-1.17,;7.31,-4.25,;7.31,-5.79,;5.98,-3.48,;4.65,-4.25,;4.65,-5.79,;3.31,-6.56,;1.98,-5.79,;1.98,-4.25,;3.31,-3.48,;.65,-6.56,;-.69,-5.79,;-.69,-4.25,;-2.02,-3.48,;-3.36,-4.25,;-3.36,-5.79,;-4.69,-6.56,;-6.02,-5.79,;-6.02,-4.25,;-7.36,-3.48,;-8.69,-4.25,;-8.69,-5.79,;-7.36,-6.56,;-10.02,-3.48,;-11.36,-4.25,;-10.02,-1.94,;-10.02,-5.02,;-2.02,-6.56,)| Show InChI InChI=1S/C25H23F3N4O2/c26-25(27,28)19-7-11-23(31-15-19)34-21-3-1-2-17(13-21)12-16-4-8-20(9-5-16)32-24(33)18-6-10-22(29)30-14-18/h1-3,6-7,10-15,20H,4-5,8-9H2,(H2,29,30)(H,32,33)/b16-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >13 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50081718
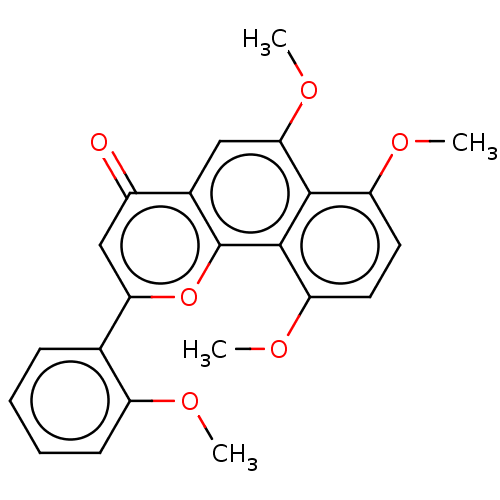
(CHEMBL3422339)Show SMILES COc1ccccc1-c1cc(=O)c2cc(OC)c3c(OC)ccc(OC)c3c2o1 Show InChI InChI=1S/C23H20O6/c1-25-16-8-6-5-7-13(16)19-12-15(24)14-11-20(28-4)21-17(26-2)9-10-18(27-3)22(21)23(14)29-19/h5-12H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 assessed as reduction in 7-ethoxyresorufin O-deethylation activity by fluorescence based EROD assay |
J Med Chem 58: 3534-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00265
BindingDB Entry DOI: 10.7270/Q2XP76NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50432672
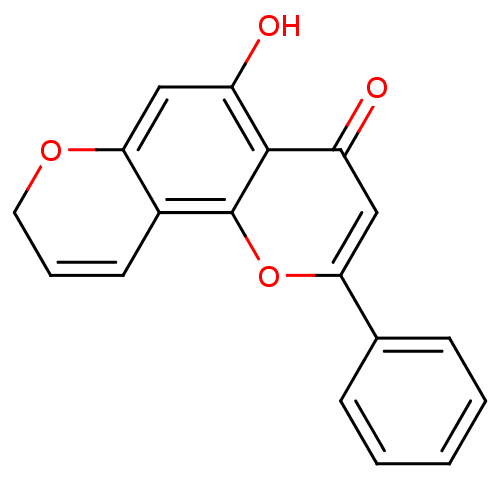
(CHEMBL2347912)Show SMILES Oc1cc2OCC=Cc2c2oc(cc(=O)c12)-c1ccccc1 |c:6| Show InChI InChI=1S/C18H12O4/c19-13-9-15(11-5-2-1-3-6-11)22-18-12-7-4-8-21-16(12)10-14(20)17(13)18/h1-7,9-10,20H,8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin)-mediated demethylation of resorufin methyl ether after 5 mins by spectrofluorimetric analysis |
J Med Chem 56: 4082-92 (2013)
Article DOI: 10.1021/jm4003654
BindingDB Entry DOI: 10.7270/Q2CN758W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50609934

(CHEMBL5287897) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM103338
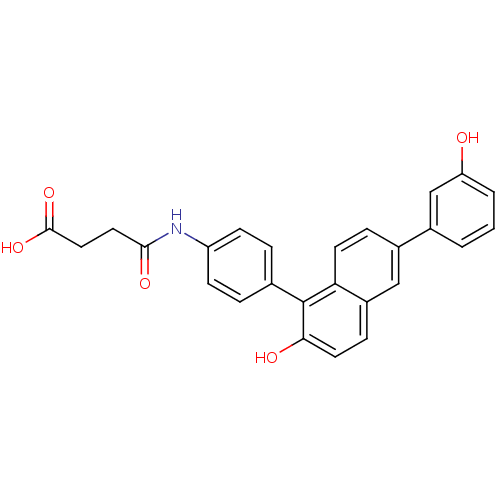
(US8546392, 59)Show SMILES OC(=O)CCC(=O)Nc1ccc(cc1)-c1c(O)ccc2cc(ccc12)-c1cccc(O)c1 Show InChI InChI=1S/C26H21NO5/c28-21-3-1-2-17(15-21)18-6-10-22-19(14-18)7-11-23(29)26(22)16-4-8-20(9-5-16)27-24(30)12-13-25(31)32/h1-11,14-15,28-29H,12-13H2,(H,27,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitaet des Saarlandes
US Patent
| Assay Description
Inhibition assay using P450 CYP enzymes. |
US Patent US8546392 (2013)
BindingDB Entry DOI: 10.7270/Q2WW7G8P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50081712
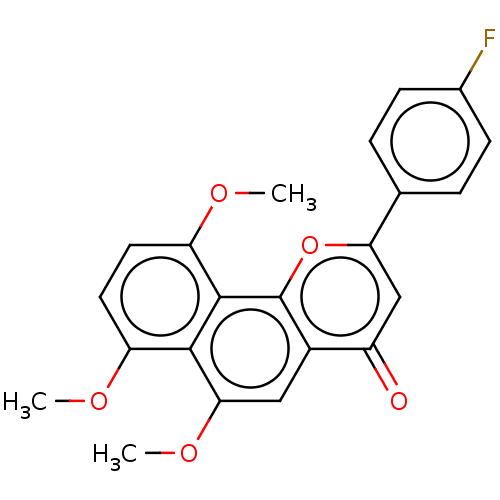
(CHEMBL3422335)Show SMILES COc1ccc(OC)c2c3oc(cc(=O)c3cc(OC)c12)-c1ccc(F)cc1 Show InChI InChI=1S/C22H17FO5/c1-25-16-8-9-17(26-2)21-20(16)19(27-3)10-14-15(24)11-18(28-22(14)21)12-4-6-13(23)7-5-12/h4-11H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 assessed as reduction in 7-ethoxyresorufin O-deethylation activity by fluorescence based EROD assay |
J Med Chem 58: 3534-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00265
BindingDB Entry DOI: 10.7270/Q2XP76NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50081709
(CHEMBL3422257)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1ccccc1 Show InChI InChI=1S/C22H18O5/c1-24-16-9-10-17(25-2)21-20(16)19(26-3)11-14-15(23)12-18(27-22(14)21)13-7-5-4-6-8-13/h4-12H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 29: 2016-2024 (2019)
Article DOI: 10.1016/j.bmcl.2019.06.040
BindingDB Entry DOI: 10.7270/Q2N01B1G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50081709

(CHEMBL3422257)Show SMILES COc1ccc(OC)c2c1c(OC)cc1c2oc(cc1=O)-c1ccccc1 Show InChI InChI=1S/C22H18O5/c1-24-16-9-10-17(25-2)21-20(16)19(26-3)11-14-15(23)12-18(27-22(14)21)13-7-5-4-6-8-13/h4-12H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 assessed as reduction in 7-ethoxyresorufin O-deethylation activity by fluorescence based EROD assay |
J Med Chem 58: 3534-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00265
BindingDB Entry DOI: 10.7270/Q2XP76NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50081720
(CHEMBL3422340)Show SMILES COc1ccc(cc1)-c1cc(=O)c2cc(OC)c3c(OC)ccc(OC)c3c2o1 Show InChI InChI=1S/C23H20O6/c1-25-14-7-5-13(6-8-14)19-12-16(24)15-11-20(28-4)21-17(26-2)9-10-18(27-3)22(21)23(15)29-19/h5-12H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 assessed as reduction in 7-ethoxyresorufin O-deethylation activity by fluorescence based EROD assay |
J Med Chem 58: 3534-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00265
BindingDB Entry DOI: 10.7270/Q2XP76NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CYP1A2 using 7-ethoxyresorufin as substrate after 50 mins in presence of NADP+ by EROD assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112895
BindingDB Entry DOI: 10.7270/Q2KW5KS3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP1A2 in human liver microsomes after 20 mins by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00500
BindingDB Entry DOI: 10.7270/Q28D00ZS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50201124
(3-(6-(1H-imidazol-1-yl)-4-methyl-1H-benzo[d]imidaz...)Show SMILES Cc1cc(cc2nc([nH]c12)-c1c(NCC(O)c2cccc(Cl)c2)cc[nH]c1=O)-n1ccnc1 Show InChI InChI=1S/C24H21ClN6O2/c1-14-9-17(31-8-7-26-13-31)11-19-22(14)30-23(29-19)21-18(5-6-27-24(21)33)28-12-20(32)15-3-2-4-16(25)10-15/h2-11,13,20,32H,12H2,1H3,(H,29,30)(H2,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Concentration required to inhibit Cytochrome P450 1A2 in vitro by 50% |
J Med Chem 48: 5639-43 (2005)
Article DOI: 10.1021/jm050392q
BindingDB Entry DOI: 10.7270/Q2MW2GPP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate in presence of glucose-6-phosphate, glucose-6-phosphate dehydrogenase and... |
Eur J Med Chem 187: (2020)
Article DOI: 10.1016/j.ejmech.2019.111938
BindingDB Entry DOI: 10.7270/Q2SX6HMM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50159614
(2-(2-Fluoro-phenyl)-benzo[h]chromen-4-one | CHEMBL...)Show InChI InChI=1S/C19H11FO2/c20-16-8-4-3-7-14(16)18-11-17(21)15-10-9-12-5-1-2-6-13(12)19(15)22-18/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP1A2 using 7-ethoxyresorufin as substrate in presence of glucose-6-phosphate, glucose-6-phosphate dehydrogenase and... |
Eur J Med Chem 187: (2020)
Article DOI: 10.1016/j.ejmech.2019.111938
BindingDB Entry DOI: 10.7270/Q2SX6HMM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50081721
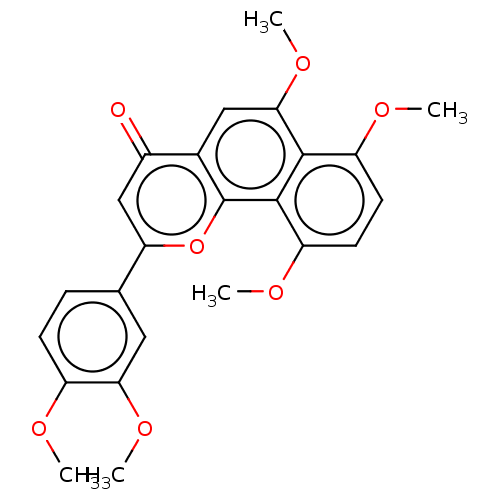
(CHEMBL3422341)Show SMILES COc1ccc(cc1OC)-c1cc(=O)c2cc(OC)c3c(OC)ccc(OC)c3c2o1 Show InChI InChI=1S/C24H22O7/c1-26-16-7-6-13(10-20(16)29-4)19-12-15(25)14-11-21(30-5)22-17(27-2)8-9-18(28-3)23(22)24(14)31-19/h6-12H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 assessed as reduction in 7-ethoxyresorufin O-deethylation activity by fluorescence based EROD assay |
J Med Chem 58: 3534-47 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00265
BindingDB Entry DOI: 10.7270/Q2XP76NJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin)-mediated demethylation of resorufin methyl ether after 5 mins by spectrofluorimetric analysis |
J Med Chem 56: 4082-92 (2013)
Article DOI: 10.1021/jm4003654
BindingDB Entry DOI: 10.7270/Q2CN758W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114569
BindingDB Entry DOI: 10.7270/Q251437D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50014323

(2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...)Show InChI InChI=1S/C19H12O2/c20-17-12-18(14-7-2-1-3-8-14)21-19-15-9-5-4-6-13(15)10-11-16(17)19/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50452363
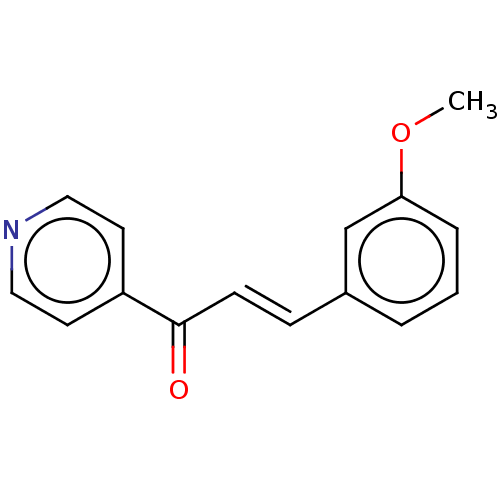
(CHEMBL4212722)Show InChI InChI=1S/C14H20NO/c1-15-9-3-5-11-10-4-2-6-14(16)12(10)7-8-13(11)15/h3,5,9-10,12,14,16H,2,4,6-8H2,1H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
De Montfort University
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 expressed in yeast microsomal membranes using 3-cyano-7-ethoxycoumarin as substrate measured after 10 mins by fluorescence... |
Bioorg Med Chem Lett 27: 5409-5414 (2017)
Article DOI: 10.1016/j.bmcl.2017.11.009
BindingDB Entry DOI: 10.7270/Q2MP55TT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50452363

(CHEMBL4212722)Show InChI InChI=1S/C14H20NO/c1-15-9-3-5-11-10-4-2-6-14(16)12(10)7-8-13(11)15/h3,5,9-10,12,14,16H,2,4,6-8H2,1H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
De Montfort University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP1A2 expressed in HEK293 cells using 3-cyano-7-ethoxycoumarin as substrate pretreated for 30 mins followed by subst... |
Bioorg Med Chem Lett 27: 5409-5414 (2017)
Article DOI: 10.1016/j.bmcl.2017.11.009
BindingDB Entry DOI: 10.7270/Q2MP55TT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data