

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM50365262 ((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM205429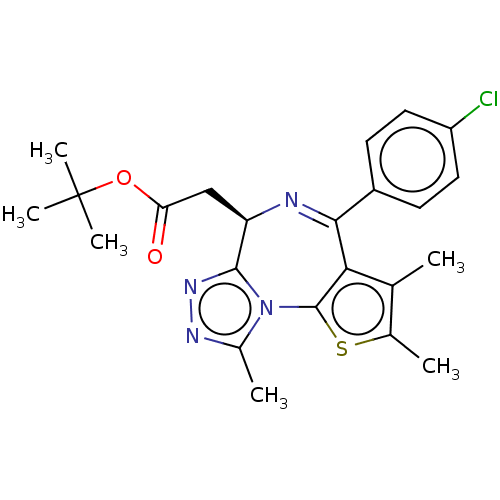 ( (R)-JQ1 (3) | US10407441, Compound (R)-JQ1 | US10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Determine the binding ability of BRD4 compounds to bromodomains of BRD2 and BRD4. 96 well plate based commercial TR-FRET Assay kits (Cayman Chemical,... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TT4V40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313777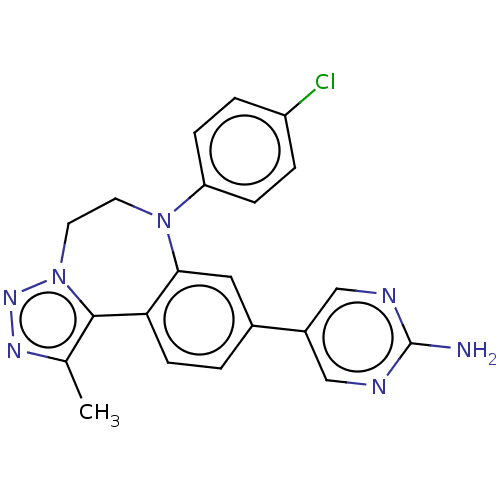 (US10167292, Example 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313778 (US10167292, Example 13) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313779 (US10167292, Example 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313784 (US10167292, Example 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313789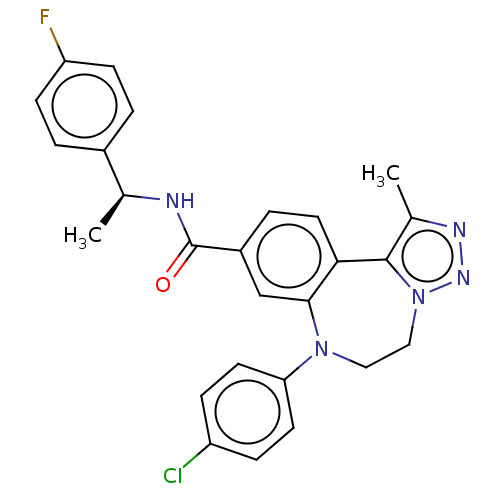 (US10167292, Example 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313791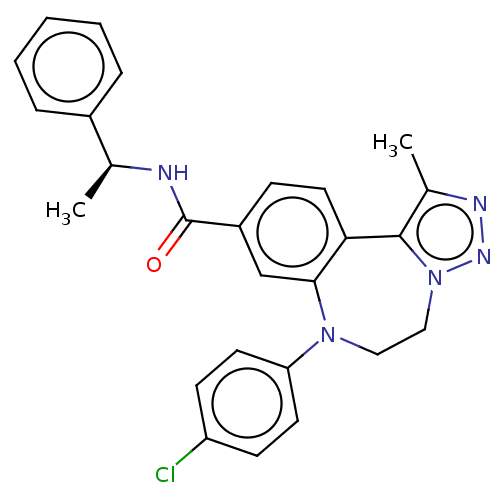 (US10167292, Example 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313792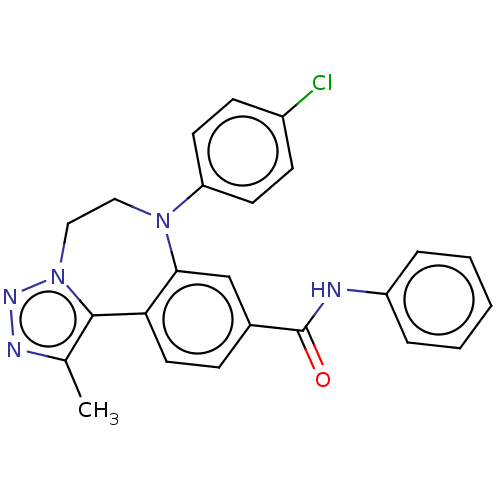 (US10167292, Example 36) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313795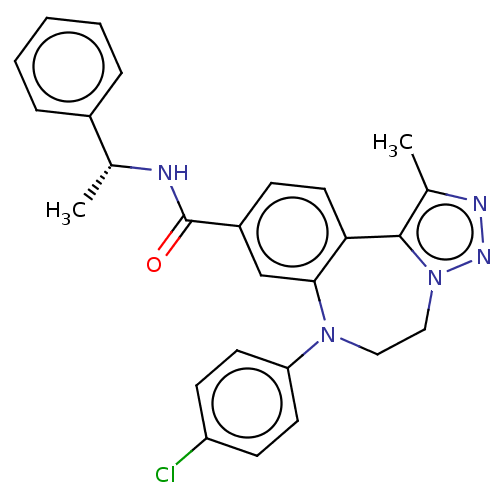 (US10167292, Example 39) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313796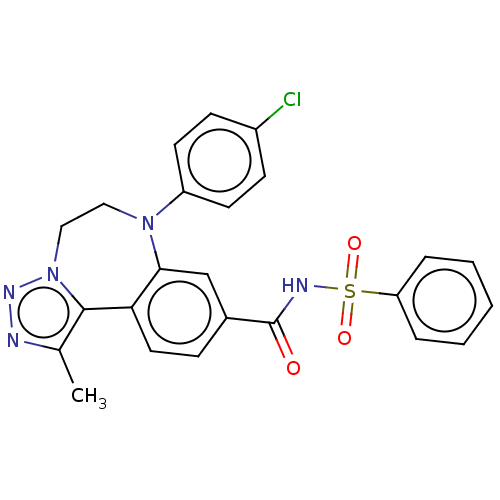 (US10167292, Example 40) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313797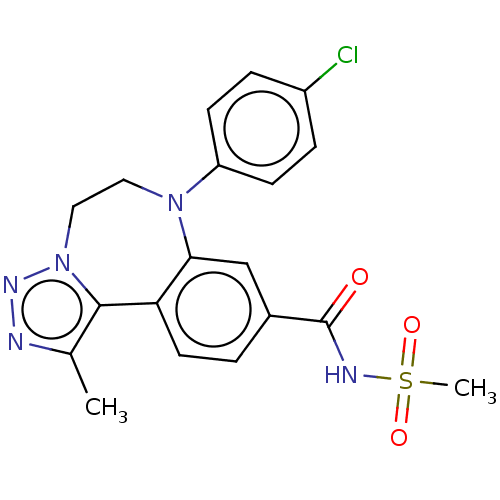 (US10167292, Example 41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313798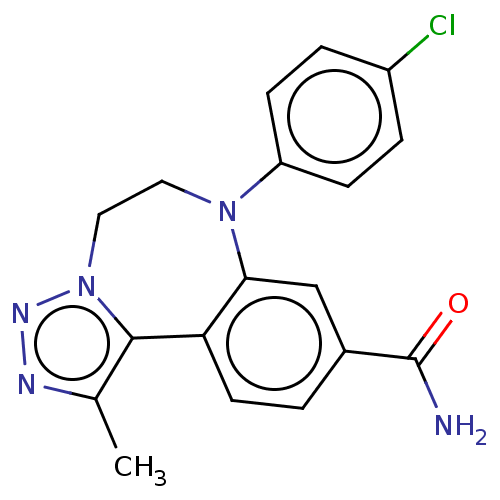 (US10167292, Example 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313799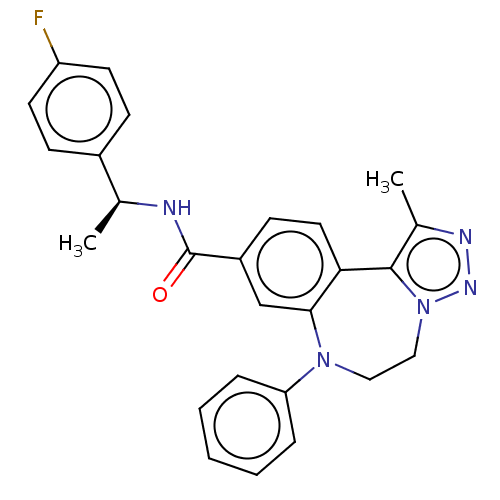 (US10167292, Example 43) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313800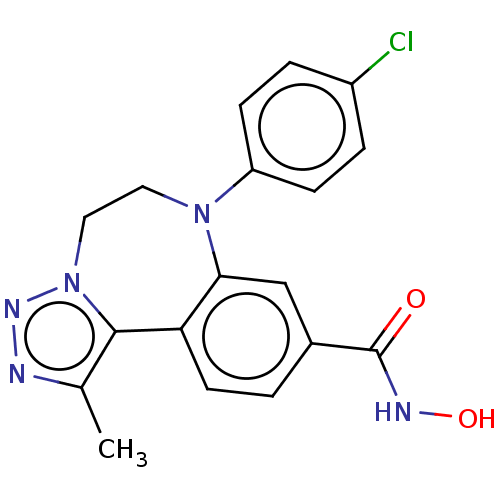 (US10167292, Example 44) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313801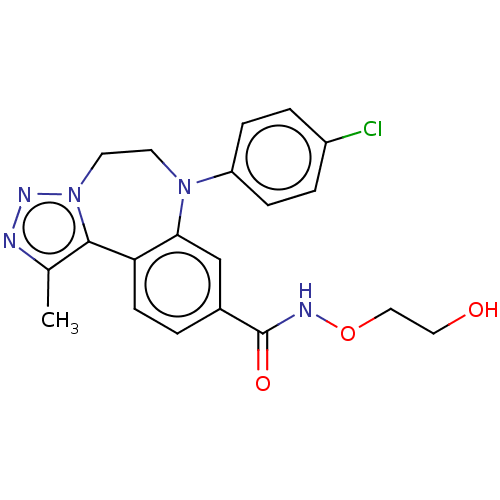 (US10167292, Example 45) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313802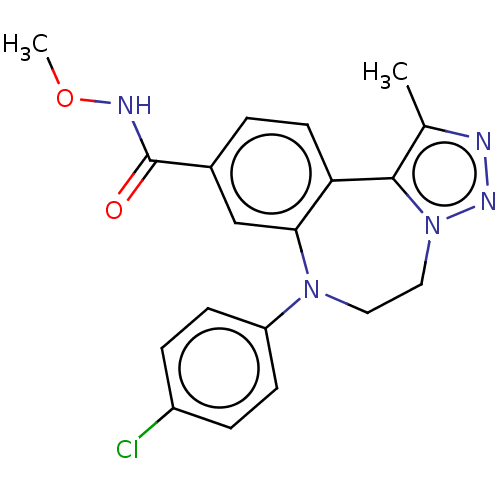 (US10167292, Example 46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313803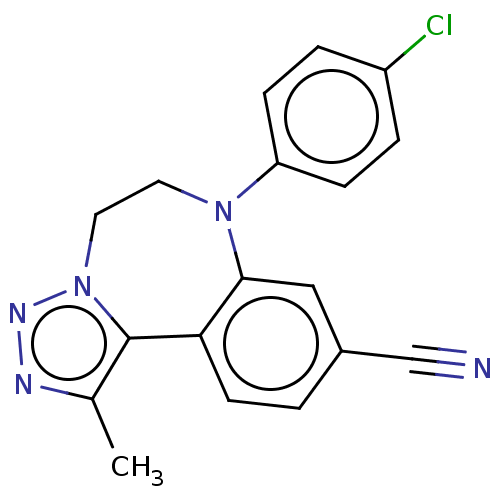 (US10167292, Example 47) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313804 (US10167292, Example 48) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313805 (US10167292, Example 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313807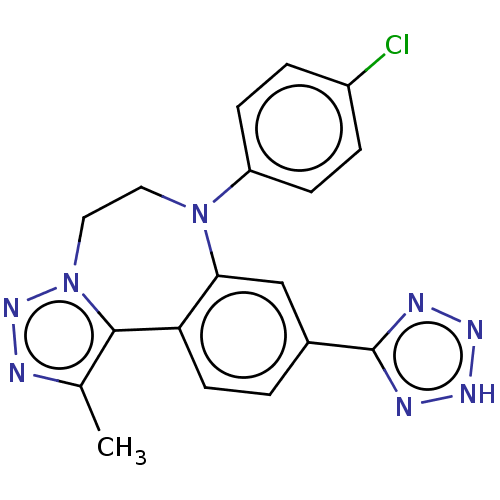 (US10167292, Example 51B) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313775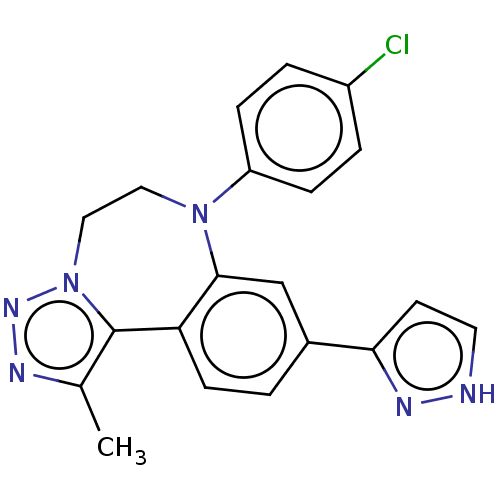 (US10167292, Example 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313773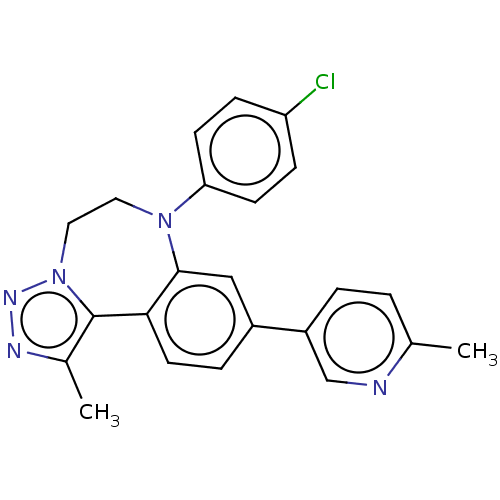 (US10167292, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313774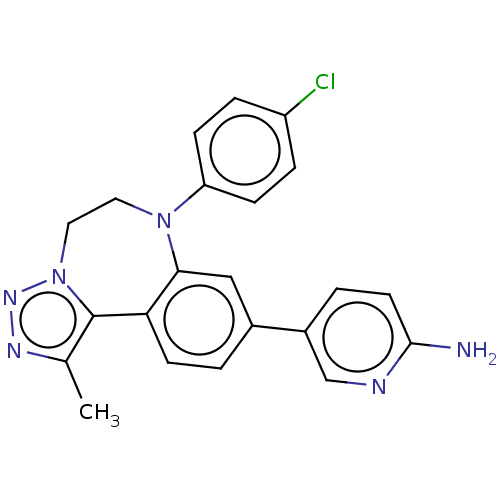 (US10167292, Example 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543570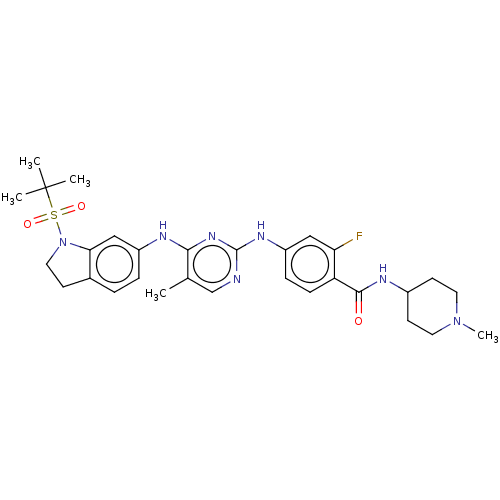 (US11279703, TABLE 6.159 | US11643396, Example SG4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543481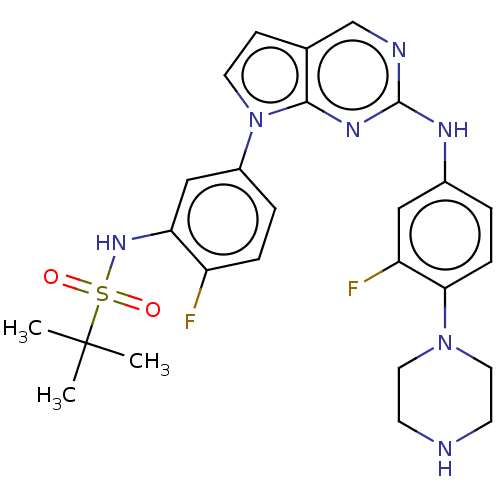 (US11279703, TABLE 6.351 | US11279703, TABLE 6.353 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543479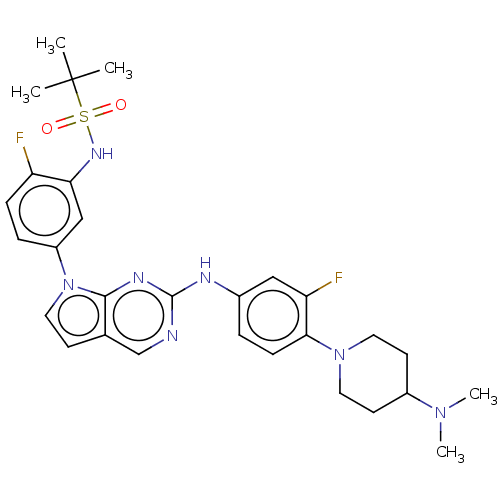 (US11279703, TABLE 6.289 | US11279703, TABLE 6.296 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543776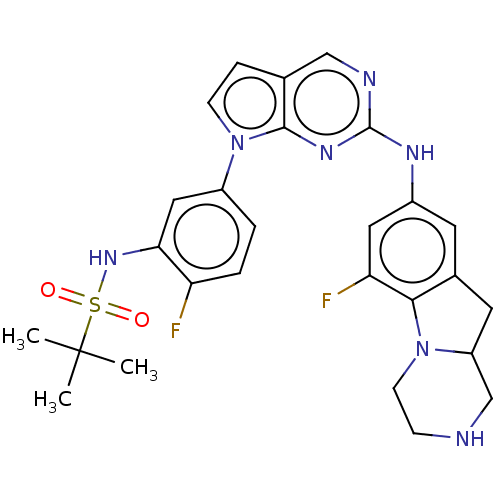 (US11279703, TABLE 6.366 | US11279703, TABLE 6.388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543569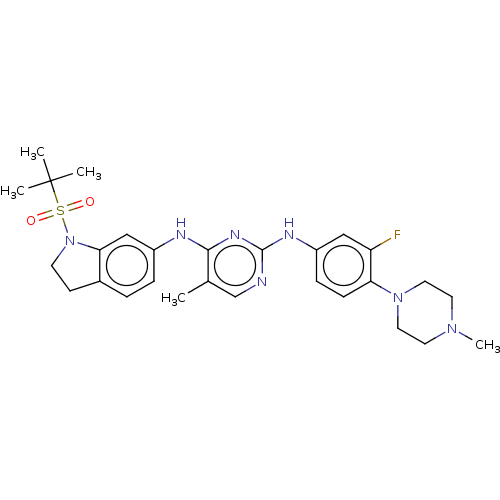 (US11279703, TABLE 6.158 | US11279703, TABLE 6.169 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543757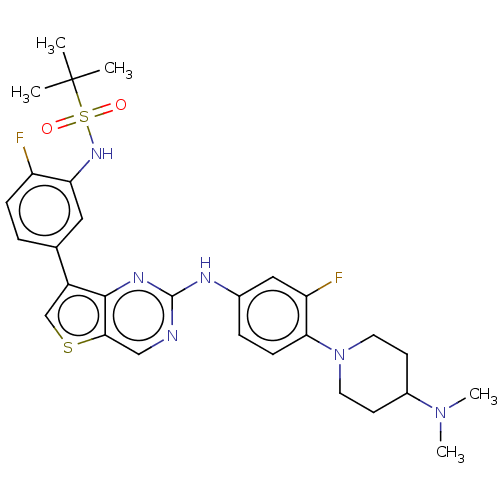 (US11279703, TABLE 6.347) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543638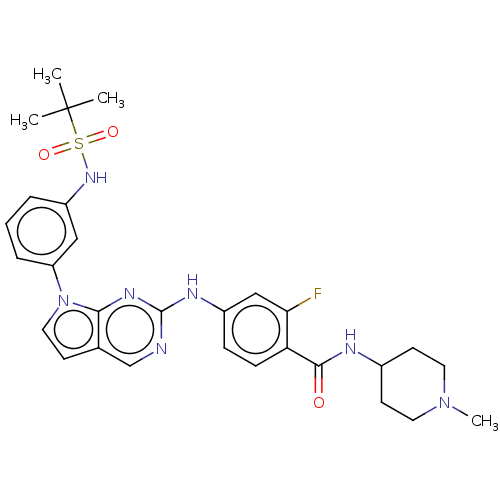 (US11279703, TABLE 6.227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 314 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543682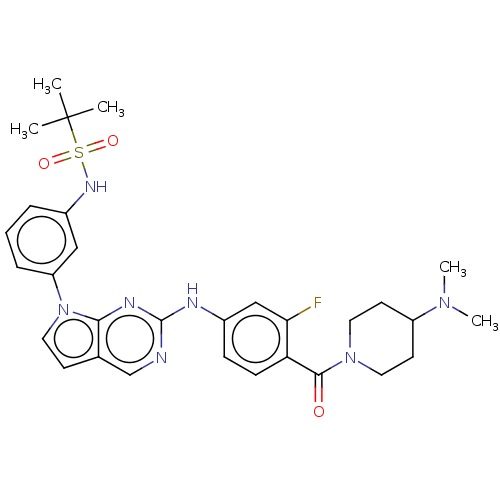 (US11279703, TABLE 6.271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 318 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543776 (US11279703, TABLE 6.366 | US11279703, TABLE 6.388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543789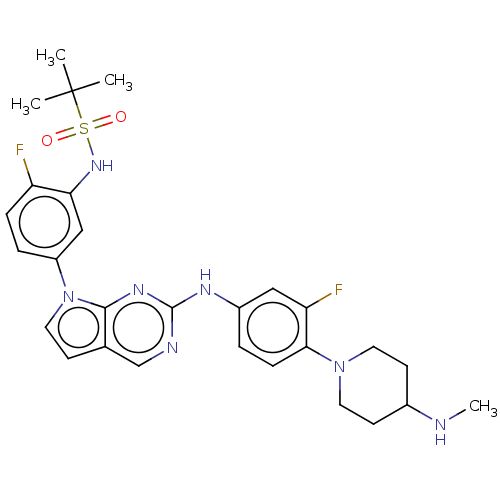 (US11279703, TABLE 6.379 | US11279703, TABLE 6.380) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543795 (US11279703, TABLE 6.385 | US11279703, TABLE 6.386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543516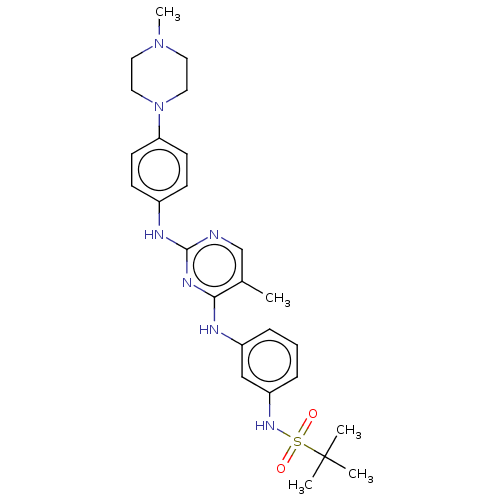 (US11279703, TABLE 6.119 | US11279703, TABLE 6.128 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 373 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543789 (US11279703, TABLE 6.379 | US11279703, TABLE 6.380) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543516 (US11279703, TABLE 6.119 | US11279703, TABLE 6.128 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543516 (US11279703, TABLE 6.119 | US11279703, TABLE 6.128 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 448 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543480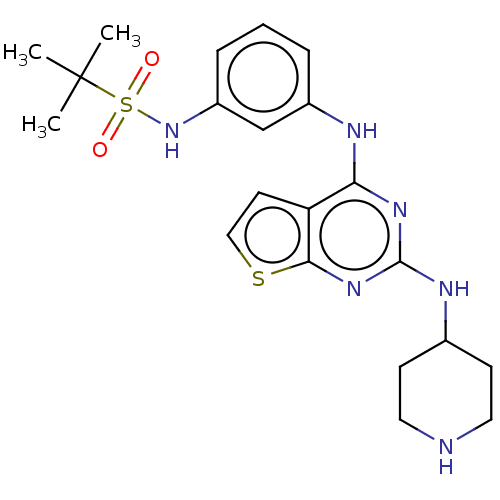 (US11279703, TABLE 6.126 | US11279703, TABLE 6.160 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 458 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543677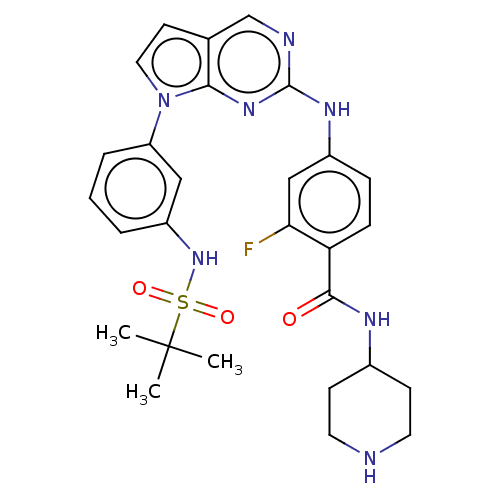 (US11279703, TABLE 6.266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 467 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543795 (US11279703, TABLE 6.385 | US11279703, TABLE 6.386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 473 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543797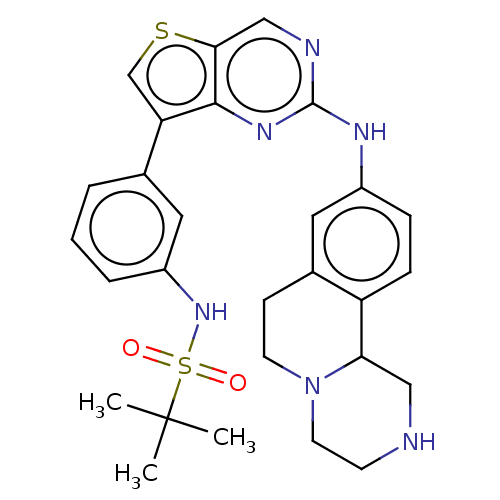 (US11279703, TABLE 6.387) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 489 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543629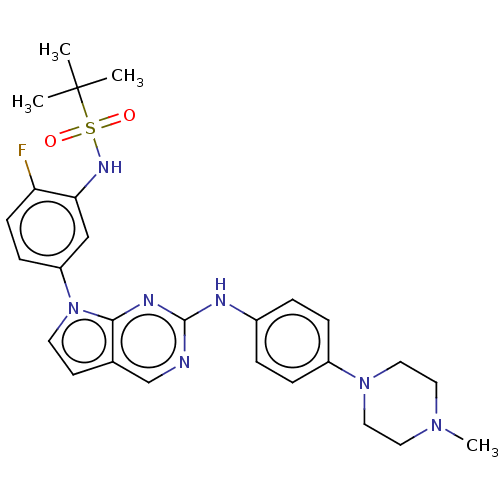 (US11279703, TABLE 6.218 | US11279703, TABLE 6.263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543756 (US11279703, TABLE 6.346) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 506 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM543684 (US11279703, TABLE 6.273) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 548 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 1. Add 20 μl of the diluted compound to each well of the 384-well opti plate2. Tap the plate gently3. Add 10 μl of the enzyme to each well ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2T43X8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313771 (US10167292, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313776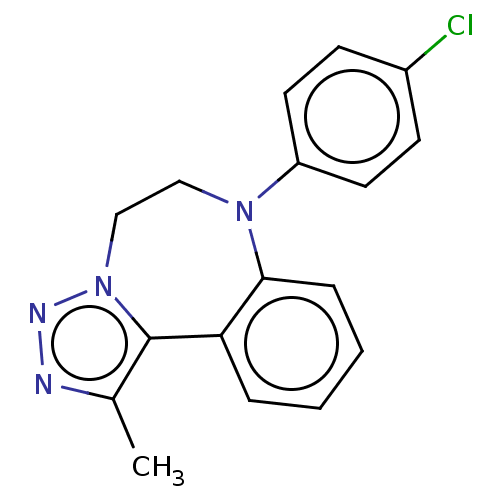 (US10167292, Example 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313780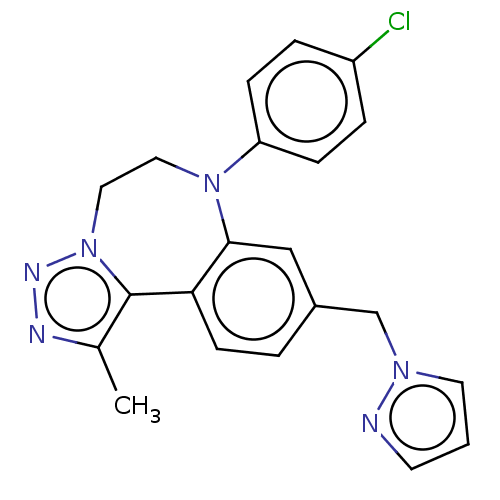 (US10167292, Example 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 [91-163] (Homo sapiens (Human)) | BDBM313781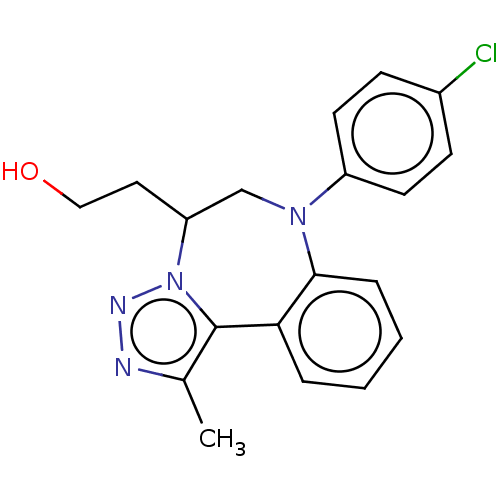 (US10167292, Example 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Catalyst Therapeutics Pty Ltd. US Patent | Assay Description BRD binding and inhibition was assessed by measuring the interaction of biotinylated acetyl-histone H4 peptide (Anaspec #64989) with the BRD target p... | US Patent US10167292 (2019) BindingDB Entry DOI: 10.7270/Q2765HFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |