 Found 1759 hits of ic50 for UniProtKB: P49682
Found 1759 hits of ic50 for UniProtKB: P49682 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337251
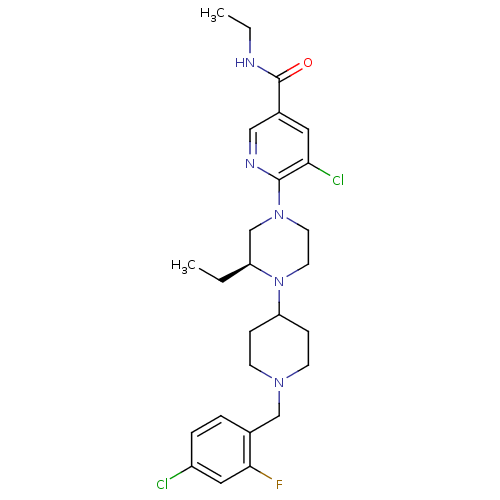
((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H34Cl2FN5O/c1-3-21-17-33(25-23(28)13-19(15-31-25)26(35)30-4-2)11-12-34(21)22-7-9-32(10-8-22)16-18-5-6-20(27)14-24(18)29/h5-6,13-15,21-22H,3-4,7-12,16-17H2,1-2H3,(H,30,35)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337251

((S)-5-chloro-6-(4-(1-(4-chloro-2-fluorobenzyl)pipe...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3F)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H34Cl2FN5O/c1-3-21-17-33(25-23(28)13-19(15-31-25)26(35)30-4-2)11-12-34(21)22-7-9-32(10-8-22)16-18-5-6-20(27)14-24(18)29/h5-6,13-15,21-22H,3-4,7-12,16-17H2,1-2H3,(H,30,35)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR3 receptor |
Bioorg Med Chem Lett 24: 1085-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.009
BindingDB Entry DOI: 10.7270/Q2BZ67JQ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50446659
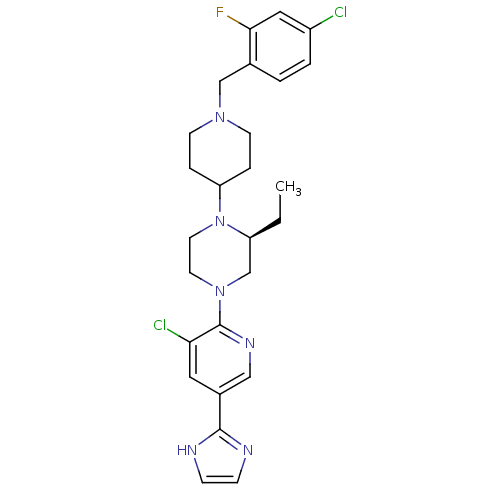
(CHEMBL3116468)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2F)CC1)c1ncc(cc1Cl)-c1ncc[nH]1 |r| Show InChI InChI=1S/C26H31Cl2FN6/c1-2-21-17-34(26-23(28)13-19(15-32-26)25-30-7-8-31-25)11-12-35(21)22-5-9-33(10-6-22)16-18-3-4-20(27)14-24(18)29/h3-4,7-8,13-15,21-22H,2,5-6,9-12,16-17H2,1H3,(H,30,31)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR3 receptor |
Bioorg Med Chem Lett 24: 1085-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.009
BindingDB Entry DOI: 10.7270/Q2BZ67JQ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358617
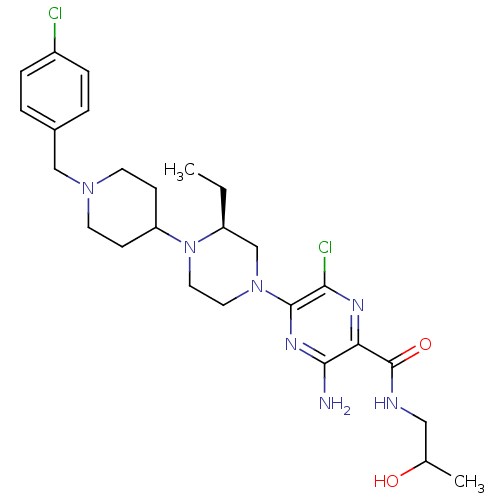
(CHEMBL1921866)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)C(=O)NCC(C)O |r| Show InChI InChI=1S/C26H37Cl2N7O2/c1-3-20-16-34(25-23(28)31-22(24(29)32-25)26(37)30-14-17(2)36)12-13-35(20)21-8-10-33(11-9-21)15-18-4-6-19(27)7-5-18/h4-7,17,20-21,36H,3,8-16H2,1-2H3,(H2,29,32)(H,30,37)/t17?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337250
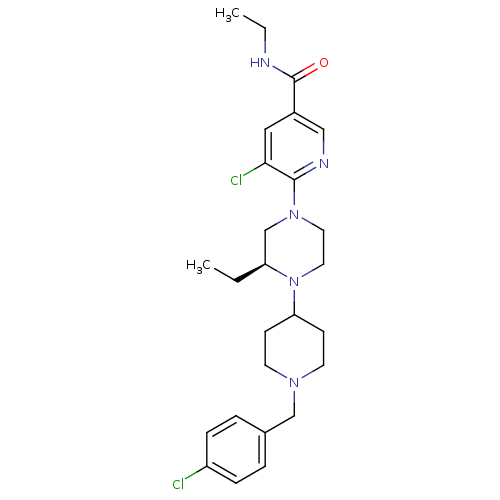
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 |
Bioorg Med Chem Lett 21: 1527-31 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.114
BindingDB Entry DOI: 10.7270/Q2CZ37FJ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358614
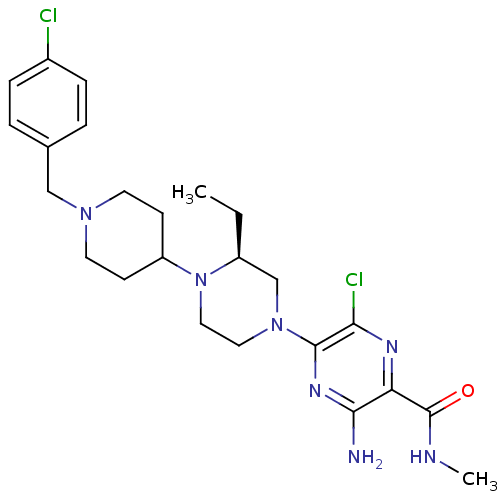
(CHEMBL1921863)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)C(=O)NC |r| Show InChI InChI=1S/C24H33Cl2N7O/c1-3-18-15-32(23-21(26)29-20(22(27)30-23)24(34)28-2)12-13-33(18)19-8-10-31(11-9-19)14-16-4-6-17(25)7-5-16/h4-7,18-19H,3,8-15H2,1-2H3,(H2,27,30)(H,28,34)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337250

((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM610449
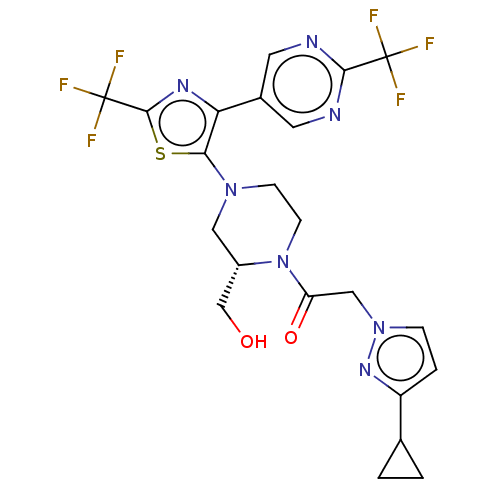
(2-(3-Cyclopropyl-pyrazol-1-yl)-1-{(S)-2-hydroxymet...)Show SMILES OC[C@@H]1CN(CCN1C(=O)Cn1ccc(n1)C1CC1)c1sc(nc1-c1cnc(nc1)C(F)(F)F)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S46X2G |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310487
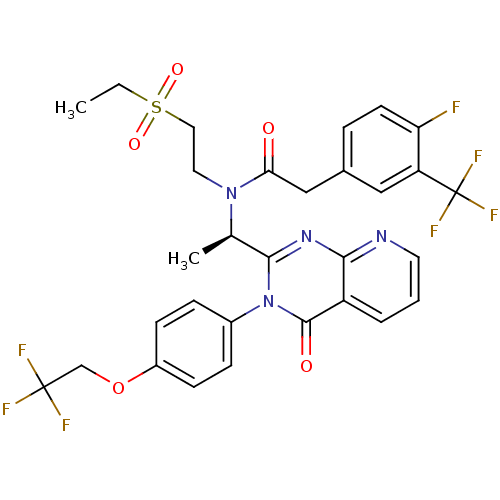
((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccc2c(=O)n1-c1ccc(OCC(F)(F)F)cc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H27F7N4O5S/c1-3-47(44,45)14-13-40(25(42)16-19-6-11-24(31)23(15-19)30(35,36)37)18(2)27-39-26-22(5-4-12-38-26)28(43)41(27)20-7-9-21(10-8-20)46-17-29(32,33)34/h4-12,15,18H,3,13-14,16-17H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human CXCR3 expressed in human PBMC assessed as inhibition of cell migration in response to IP10 in buffer |
Bioorg Med Chem Lett 19: 5114-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.032
BindingDB Entry DOI: 10.7270/Q2FX79KZ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358616
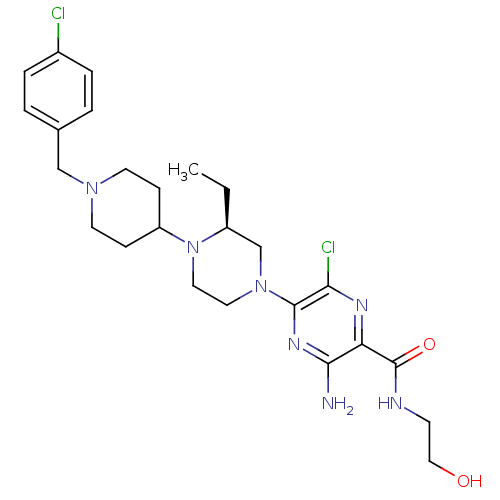
(CHEMBL1921865)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)C(=O)NCCO |r| Show InChI InChI=1S/C25H35Cl2N7O2/c1-2-19-16-33(24-22(27)30-21(23(28)31-24)25(36)29-9-14-35)12-13-34(19)20-7-10-32(11-8-20)15-17-3-5-18(26)6-4-17/h3-6,19-20,35H,2,7-16H2,1H3,(H2,28,31)(H,29,36)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50371494

(CHEMBL256589)Show SMILES CCOc1ccc(cc1)-n1cc(nc1[C@@H](C)N(CCS(=O)(=O)CC)C(=O)Cc1ccc(F)c(c1)C(F)(F)F)-c1ccccc1 Show InChI InChI=1S/C32H33F4N3O4S/c1-4-43-26-14-12-25(13-15-26)39-21-29(24-9-7-6-8-10-24)37-31(39)22(3)38(17-18-44(41,42)5-2)30(40)20-23-11-16-28(33)27(19-23)32(34,35)36/h6-16,19,21-22H,4-5,17-18,20H2,1-3H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from human recombinant CXCR3 receptor expressed in IL2-activated human PBMC |
Bioorg Med Chem Lett 18: 608-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.072
BindingDB Entry DOI: 10.7270/Q2668F1D |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50446651
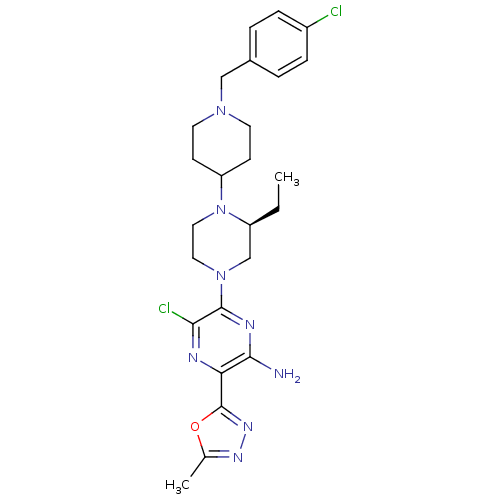
(CHEMBL3116476)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)-c1nnc(C)o1 |r| Show InChI InChI=1S/C25H32Cl2N8O/c1-3-19-15-34(24-22(27)29-21(23(28)30-24)25-32-31-16(2)36-25)12-13-35(19)20-8-10-33(11-9-20)14-17-4-6-18(26)7-5-17/h4-7,19-20H,3,8-15H2,1-2H3,(H2,28,30)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR3 receptor |
Bioorg Med Chem Lett 24: 1085-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.009
BindingDB Entry DOI: 10.7270/Q2BZ67JQ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM610445
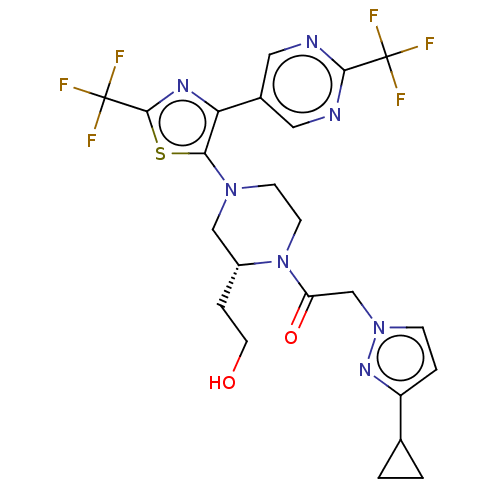
(2-(3-Cyclopropyl-pyrazol-1-yl)-1-{(R)-2-(2-hydroxy...)Show SMILES OCC[C@@H]1CN(CCN1C(=O)Cn1ccc(n1)C1CC1)c1sc(nc1-c1cnc(nc1)C(F)(F)F)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S46X2G |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310487

((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccc2c(=O)n1-c1ccc(OCC(F)(F)F)cc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H27F7N4O5S/c1-3-47(44,45)14-13-40(25(42)16-19-6-11-24(31)23(15-19)30(35,36)37)18(2)27-39-26-22(5-4-12-38-26)28(43)41(27)20-7-9-21(10-8-20)46-17-29(32,33)34/h4-12,15,18H,3,13-14,16-17H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human CXCR3 expressed in human PBMC assessed as inhibition of cell migration in response to MIG in buffer |
Bioorg Med Chem Lett 19: 5114-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.032
BindingDB Entry DOI: 10.7270/Q2FX79KZ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229439
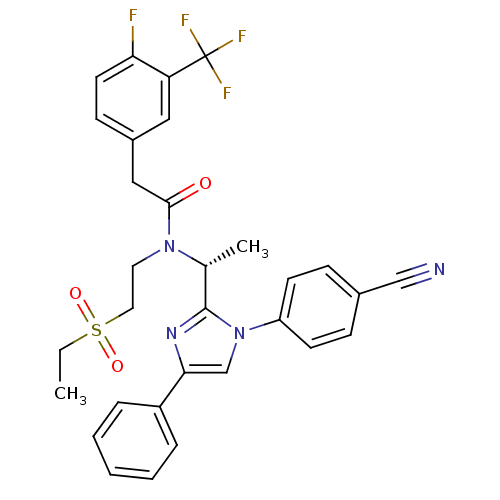
((R)-N-(1-(1-(4-cyanophenyl)-4-phenyl-1H-imidazol-2...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc(cn1-c1ccc(cc1)C#N)-c1ccccc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C31H28F4N4O3S/c1-3-43(41,42)16-15-38(29(40)18-23-11-14-27(32)26(17-23)31(33,34)35)21(2)30-37-28(24-7-5-4-6-8-24)20-39(30)25-12-9-22(19-36)10-13-25/h4-14,17,20-21H,3,15-16,18H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from human recombinant CXCR3 receptor expressed in IL2-activated human PBMC |
Bioorg Med Chem Lett 18: 608-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.072
BindingDB Entry DOI: 10.7270/Q2668F1D |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM228190
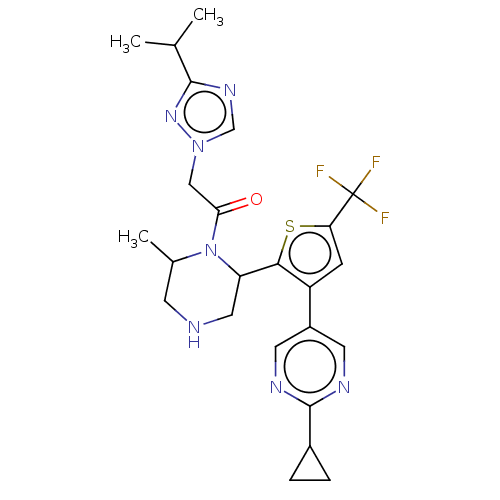
(1-{(R)-4-[4-(2-Cyclopropyl-pyrimidin-5-yl)-2-trifl...)Show SMILES CC(C)c1ncn(CC(=O)N2C(C)CNCC2c2sc(cc2-c2cnc(nc2)C2CC2)C(F)(F)F)n1 Show InChI InChI=1S/C24H28F3N7OS/c1-13(2)22-31-12-33(32-22)11-20(35)34-14(3)7-28-10-18(34)21-17(6-19(36-21)24(25,26)27)16-8-29-23(30-9-16)15-4-5-15/h6,8-9,12-15,18,28H,4-5,7,10-11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | n/a |
IDORSIA PHARMACEUTICALS LTD.
US Patent
| Assay Description
The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the... |
US Patent US10047080 (2018)
BindingDB Entry DOI: 10.7270/Q2JQ130F |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229439

((R)-N-(1-(1-(4-cyanophenyl)-4-phenyl-1H-imidazol-2...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc(cn1-c1ccc(cc1)C#N)-c1ccccc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C31H28F4N4O3S/c1-3-43(41,42)16-15-38(29(40)18-23-11-14-27(32)26(17-23)31(33,34)35)21(2)30-37-28(24-7-5-4-6-8-24)20-39(30)25-12-9-22(19-36)10-13-25/h4-14,17,20-21H,3,15-16,18H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM610418
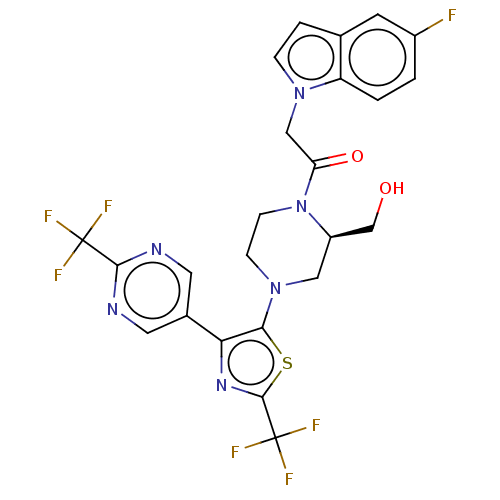
(2-(5-Fluoro-indol-1-yl)-1-{(R)-2-hydroxymethyl-4-[...)Show SMILES OC[C@H]1CN(CCN1C(=O)Cn1ccc2cc(F)ccc12)c1sc(nc1-c1cnc(nc1)C(F)(F)F)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S46X2G |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310487

((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccc2c(=O)n1-c1ccc(OCC(F)(F)F)cc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H27F7N4O5S/c1-3-47(44,45)14-13-40(25(42)16-19-6-11-24(31)23(15-19)30(35,36)37)18(2)27-39-26-22(5-4-12-38-26)28(43)41(27)20-7-9-21(10-8-20)46-17-29(32,33)34/h4-12,15,18H,3,13-14,16-17H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human CXCR3 expressed in human PBMC assessed as inhibition of cell migration in response to ITAC in RPMI buffer |
Bioorg Med Chem Lett 19: 5114-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.032
BindingDB Entry DOI: 10.7270/Q2FX79KZ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358609
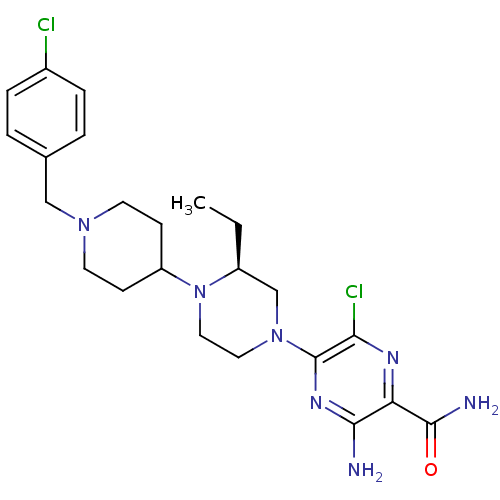
(CHEMBL1921858)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)C(N)=O |r| Show InChI InChI=1S/C23H31Cl2N7O/c1-2-17-14-31(23-20(25)28-19(22(27)33)21(26)29-23)11-12-32(17)18-7-9-30(10-8-18)13-15-3-5-16(24)6-4-15/h3-6,17-18H,2,7-14H2,1H3,(H2,26,29)(H2,27,33)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358615
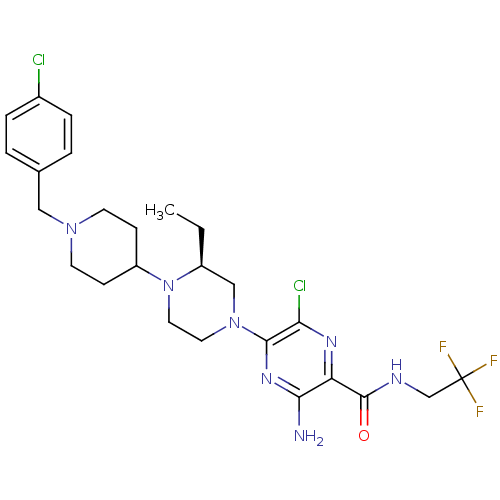
(CHEMBL1921864)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)C(=O)NCC(F)(F)F |r| Show InChI InChI=1S/C25H32Cl2F3N7O/c1-2-18-14-36(23-21(27)33-20(22(31)34-23)24(38)32-15-25(28,29)30)11-12-37(18)19-7-9-35(10-8-19)13-16-3-5-17(26)6-4-16/h3-6,18-19H,2,7-15H2,1H3,(H2,31,34)(H,32,38)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229381
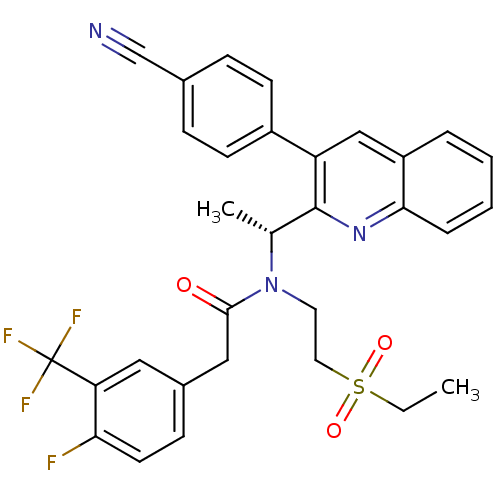
((R)-N-(1-(3-(4-cyanophenyl)quinolin-2-yl)ethyl)-N-...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ccccc2cc1-c1ccc(cc1)C#N)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C31H27F4N3O3S/c1-3-42(40,41)15-14-38(29(39)17-22-10-13-27(32)26(16-22)31(33,34)35)20(2)30-25(23-11-8-21(19-36)9-12-23)18-24-6-4-5-7-28(24)37-30/h4-13,16,18,20H,3,14-15,17H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-IP-10 from CXCR3 receptor expressed in human PBMC in RPMI-1640 buffer supplemented with 0.5% BSA |
Bioorg Med Chem Lett 18: 688-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.060
BindingDB Entry DOI: 10.7270/Q2WQ03HW |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358609

(CHEMBL1921858)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)C(N)=O |r| Show InChI InChI=1S/C23H31Cl2N7O/c1-2-17-14-31(23-20(25)28-19(22(27)33)21(26)29-23)11-12-32(17)18-7-9-30(10-8-18)13-15-3-5-16(24)6-4-15/h3-6,17-18H,2,7-14H2,1H3,(H2,26,29)(H2,27,33)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR3 receptor |
Bioorg Med Chem Lett 24: 1085-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.009
BindingDB Entry DOI: 10.7270/Q2BZ67JQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50446640
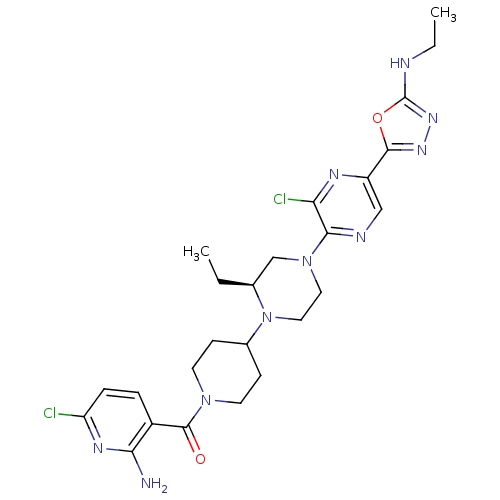
(CHEMBL3116487)Show SMILES CCNc1nnc(o1)-c1cnc(N2CCN([C@@H](CC)C2)C2CCN(CC2)C(=O)c2ccc(Cl)nc2N)c(Cl)n1 |r| Show InChI InChI=1S/C25H32Cl2N10O2/c1-3-15-14-36(22-20(27)31-18(13-30-22)23-33-34-25(39-23)29-4-2)11-12-37(15)16-7-9-35(10-8-16)24(38)17-5-6-19(26)32-21(17)28/h5-6,13,15-16H,3-4,7-12,14H2,1-2H3,(H2,28,32)(H,29,34)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR3 receptor |
Bioorg Med Chem Lett 24: 1085-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.009
BindingDB Entry DOI: 10.7270/Q2BZ67JQ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM389791

(2-Imidazo[4,5-b]pyridin-3-yl-1-{(R)-2-methyl-4-[2-...)Show SMILES CCCc1ncc(cn1)N1CCc2cccc(N3CCN([C@H](C)C3)C(=O)Cn3cnc4cccnc34)c2C1 Show InChI InChI=1S/C29H34N8O/c1-3-6-27-31-15-23(16-32-27)34-12-10-22-7-4-9-26(24(22)18-34)35-13-14-37(21(2)17-35)28(38)19-36-20-33-25-8-5-11-30-29(25)36/h4-5,7-9,11,15-16,20-21H,3,6,10,12-14,17-19H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA
| Assay Description
The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the... |
Bioorg Med Chem Lett 17: 3473-9 (2007)
BindingDB Entry DOI: 10.7270/Q20R9RRK |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50300899
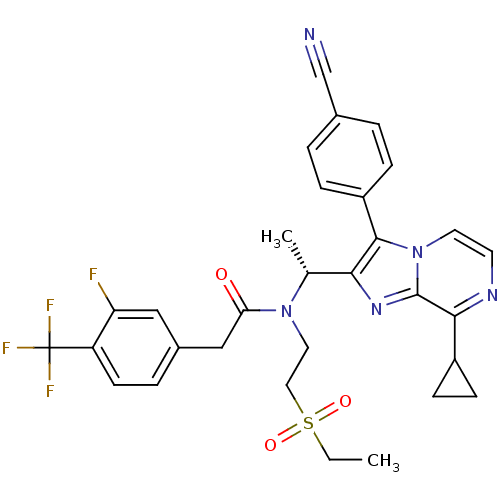
((R)-N-(1-(3-(4-cyanophenyl)-8-cyclopropylimidazo[1...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2c(nccn2c1-c1ccc(cc1)C#N)C1CC1)C(=O)Cc1ccc(c(F)c1)C(F)(F)F |r| Show InChI InChI=1S/C31H29F4N5O3S/c1-3-44(42,43)15-14-39(26(41)17-21-6-11-24(25(32)16-21)31(33,34)35)19(2)27-29(23-7-4-20(18-36)5-8-23)40-13-12-37-28(22-9-10-22)30(40)38-27/h4-8,11-13,16,19,22H,3,9-10,14-15,17H2,1-2H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50300900
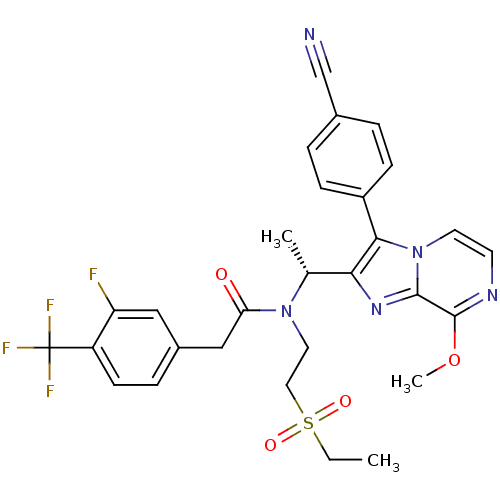
((R)-N-(1-(3-(4-cyanophenyl)-8-methoxyimidazo[1,2-a...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2c(OC)nccn2c1-c1ccc(cc1)C#N)C(=O)Cc1ccc(c(F)c1)C(F)(F)F |r| Show InChI InChI=1S/C29H27F4N5O4S/c1-4-43(40,41)14-13-37(24(39)16-20-7-10-22(23(30)15-20)29(31,32)33)18(2)25-26(21-8-5-19(17-34)6-9-21)38-12-11-35-28(42-3)27(38)36-25/h5-12,15,18H,4,13-14,16H2,1-3H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM228186

(2-[3-(1-Hydroxy-ethyl)-[1,2,4]triazol-1-yl]-1-{(R)...)Show SMILES CC1CNCC(N1C(=O)Cn1cnc(CCO)n1)c1sc(cc1-c1cnc(nc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H21F6N7O2S/c1-11-5-28-8-14(34(11)17(36)9-33-10-31-16(32-33)2-3-35)18-13(4-15(37-18)20(22,23)24)12-6-29-19(30-7-12)21(25,26)27/h4,6-7,10-11,14,28,35H,2-3,5,8-9H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.4 | n/a |
IDORSIA PHARMACEUTICALS LTD.
US Patent
| Assay Description
The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the... |
US Patent US10047080 (2018)
BindingDB Entry DOI: 10.7270/Q2JQ130F |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358630
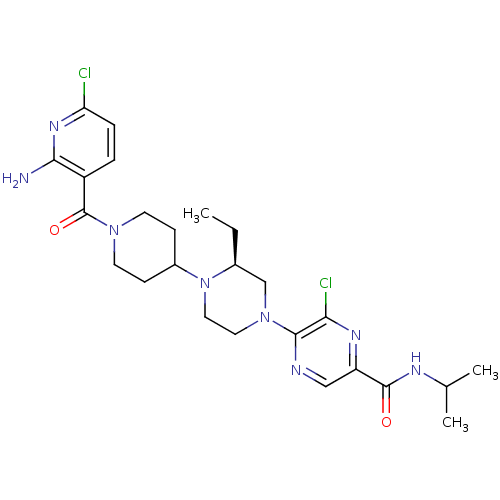
(CHEMBL1921879)Show SMILES CC[C@H]1CN(CCN1C1CCN(CC1)C(=O)c1ccc(Cl)nc1N)c1ncc(nc1Cl)C(=O)NC(C)C |r| Show InChI InChI=1S/C25H34Cl2N8O2/c1-4-16-14-34(23-21(27)31-19(13-29-23)24(36)30-15(2)3)11-12-35(16)17-7-9-33(10-8-17)25(37)18-5-6-20(26)32-22(18)28/h5-6,13,15-17H,4,7-12,14H2,1-3H3,(H2,28,32)(H,30,36)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50300907
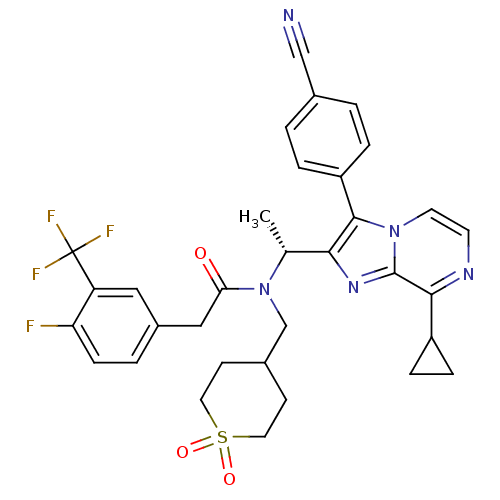
(CHEMBL576097 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...)Show SMILES C[C@@H](N(CC1CCS(=O)(=O)CC1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F)c1nc2c(nccn2c1-c1ccc(cc1)C#N)C1CC1 |r| Show InChI InChI=1S/C33H31F4N5O3S/c1-20(29-31(25-5-2-21(18-38)3-6-25)41-13-12-39-30(24-7-8-24)32(41)40-29)42(19-22-10-14-46(44,45)15-11-22)28(43)17-23-4-9-27(34)26(16-23)33(35,36)37/h2-6,9,12-13,16,20,22,24H,7-8,10-11,14-15,17,19H2,1H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358619
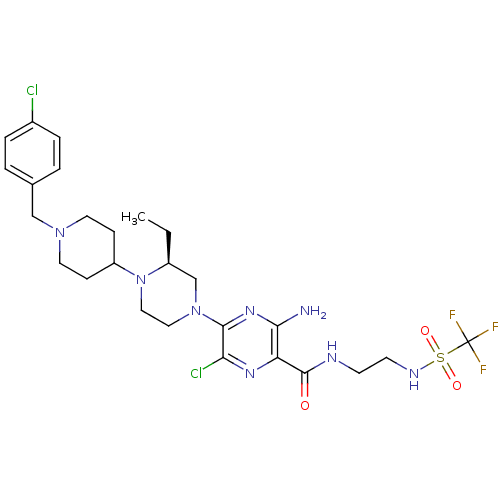
(CHEMBL1921868)Show SMILES CC[C@H]1CN(CCN1C1CCN(Cc2ccc(Cl)cc2)CC1)c1nc(N)c(nc1Cl)C(=O)NCCNS(=O)(=O)C(F)(F)F |r| Show InChI InChI=1S/C26H35Cl2F3N8O3S/c1-2-19-16-38(13-14-39(19)20-7-11-37(12-8-20)15-17-3-5-18(27)6-4-17)24-22(28)35-21(23(32)36-24)25(40)33-9-10-34-43(41,42)26(29,30)31/h3-6,19-20,34H,2,7-16H2,1H3,(H2,32,36)(H,33,40)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50300905
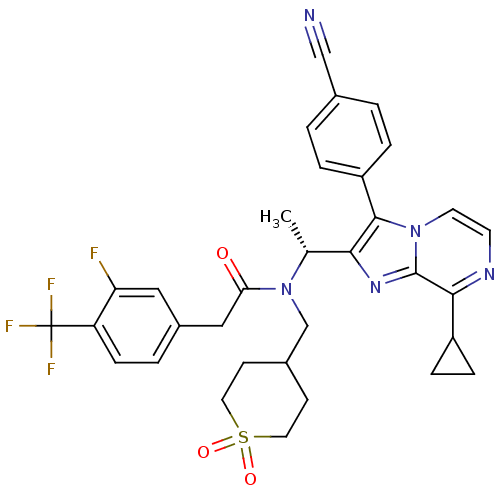
(CHEMBL578192 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...)Show SMILES C[C@@H](N(CC1CCS(=O)(=O)CC1)C(=O)Cc1ccc(c(F)c1)C(F)(F)F)c1nc2c(nccn2c1-c1ccc(cc1)C#N)C1CC1 |r| Show InChI InChI=1S/C33H31F4N5O3S/c1-20(29-31(25-5-2-21(18-38)3-6-25)41-13-12-39-30(24-7-8-24)32(41)40-29)42(19-22-10-14-46(44,45)15-11-22)28(43)17-23-4-9-26(27(34)16-23)33(35,36)37/h2-6,9,12-13,16,20,22,24H,7-8,10-11,14-15,17,19H2,1H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM389773
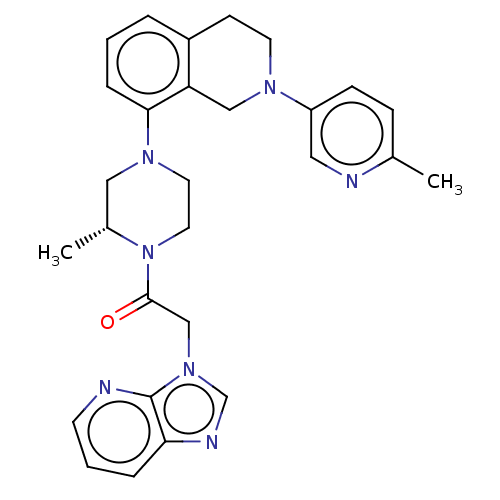
(US9951063, 92)Show SMILES C[C@@H]1CN(CCN1C(=O)Cn1cnc2cccnc12)c1cccc2CCN(Cc12)c1ccc(C)nc1 Show InChI InChI=1S/C28H31N7O/c1-20-8-9-23(15-30-20)32-12-10-22-5-3-7-26(24(22)17-32)33-13-14-35(21(2)16-33)27(36)18-34-19-31-25-6-4-11-29-28(25)34/h3-9,11,15,19,21H,10,12-14,16-18H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Tanabe Research Laboratories USA
| Assay Description
The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the... |
Bioorg Med Chem Lett 17: 3473-9 (2007)
BindingDB Entry DOI: 10.7270/Q20R9RRK |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310483

((R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydropyri...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(C1CCN(CC1)C(C)C)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C34H37F4N5O3/c1-5-46-26-11-9-24(10-12-26)43-32(40-31-27(33(43)45)7-6-16-39-31)22(4)42(25-14-17-41(18-15-25)21(2)3)30(44)20-23-8-13-29(35)28(19-23)34(36,37)38/h6-13,16,19,21-22,25H,5,14-15,17-18,20H2,1-4H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1P10 from human CXCR3 expressed in PBMC after 2 hrs in RPMI buffer by scintillation counting |
Bioorg Med Chem Lett 19: 5114-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.032
BindingDB Entry DOI: 10.7270/Q2FX79KZ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM228176

(2-(3-tert-Butyl-[1,2,4]triazol-1-yl)-1-{(R)-4-[4-(...)Show SMILES CCOc1ncc(cn1)-c1cc(sc1C1CNCC(C)N1C(=O)Cn1cnc(n1)C(C)(C)C)C(F)(F)F Show InChI InChI=1S/C24H30F3N7O2S/c1-6-36-22-29-9-15(10-30-22)16-7-18(24(25,26)27)37-20(16)17-11-28-8-14(2)34(17)19(35)12-33-13-31-21(32-33)23(3,4)5/h7,9-10,13-14,17,28H,6,8,11-12H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | n/a |
IDORSIA PHARMACEUTICALS LTD.
US Patent
| Assay Description
The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the... |
US Patent US10047080 (2018)
BindingDB Entry DOI: 10.7270/Q2JQ130F |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50446641
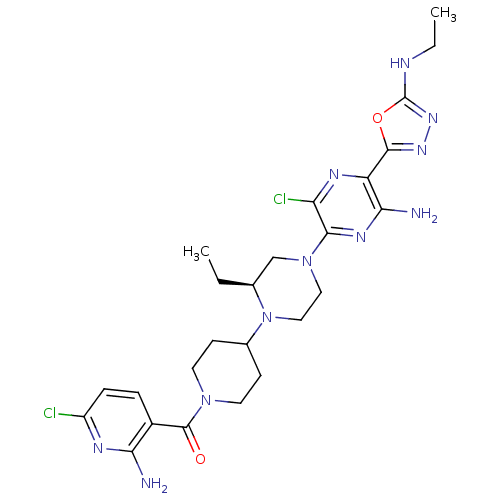
(CHEMBL3116486)Show SMILES CCNc1nnc(o1)-c1nc(Cl)c(nc1N)N1CCN([C@@H](CC)C1)C1CCN(CC1)C(=O)c1ccc(Cl)nc1N |r| Show InChI InChI=1S/C25H33Cl2N11O2/c1-3-14-13-37(22-19(27)32-18(21(29)33-22)23-34-35-25(40-23)30-4-2)11-12-38(14)15-7-9-36(10-8-15)24(39)16-5-6-17(26)31-20(16)28/h5-6,14-15H,3-4,7-13H2,1-2H3,(H2,28,31)(H2,29,33)(H,30,35)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR3 receptor |
Bioorg Med Chem Lett 24: 1085-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.009
BindingDB Entry DOI: 10.7270/Q2BZ67JQ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50229462

((R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydroquin...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ccccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C33H28F4N4O3/c1-3-44-25-13-11-24(12-14-25)41-31(39-29-9-5-4-8-26(29)32(41)43)21(2)40(20-23-7-6-16-38-19-23)30(42)18-22-10-15-28(34)27(17-22)33(35,36)37/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from human recombinant CXCR3 receptor expressed in IL2-activated human PBMC |
Bioorg Med Chem Lett 18: 608-13 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.072
BindingDB Entry DOI: 10.7270/Q2668F1D |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310487

((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccc2c(=O)n1-c1ccc(OCC(F)(F)F)cc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H27F7N4O5S/c1-3-47(44,45)14-13-40(25(42)16-19-6-11-24(31)23(15-19)30(35,36)37)18(2)27-39-26-22(5-4-12-38-26)28(43)41(27)20-7-9-21(10-8-20)46-17-29(32,33)34/h4-12,15,18H,3,13-14,16-17H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-ITAC from human CXCR3 expressed in human PBMC after 2 hrs in RPMI buffer by scintillation counting |
Bioorg Med Chem Lett 19: 5114-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.032
BindingDB Entry DOI: 10.7270/Q2FX79KZ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM610446

(1-{(S)-2-Hydroxymethyl-4-[2-trifluoromethyl-4-(2-t...)Show SMILES Cc1ccn(CC(=O)N2CCN(C[C@H]2CO)c2sc(nc2-c2cnc(nc2)C(F)(F)F)C(F)(F)F)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S46X2G |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310486

((R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydropyri...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(CCS(=O)(=O)CC)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H30F4N4O5S/c1-4-43-22-11-9-21(10-12-22)38-28(36-27-23(29(38)40)7-6-14-35-27)19(3)37(15-16-44(41,42)5-2)26(39)18-20-8-13-25(31)24(17-20)30(32,33)34/h6-14,17,19H,4-5,15-16,18H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1P10 from human CXCR3 expressed in PBMC after 2 hrs in RPMI buffer by scintillation counting |
Bioorg Med Chem Lett 19: 5114-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.032
BindingDB Entry DOI: 10.7270/Q2FX79KZ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM610443
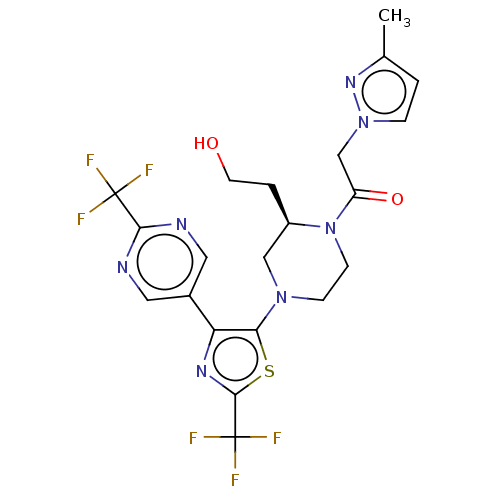
(1-{(R)-2-(2-Hydroxy-ethyl)-4-[2-trifluoromethyl-4-...)Show SMILES Cc1ccn(CC(=O)N2CCN(C[C@H]2CCO)c2sc(nc2-c2cnc(nc2)C(F)(F)F)C(F)(F)F)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S46X2G |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310487

((R)-N-(2-(ethylsulfonyl)ethyl)-2-(4-fluoro-3-(trif...)Show SMILES CCS(=O)(=O)CCN([C@H](C)c1nc2ncccc2c(=O)n1-c1ccc(OCC(F)(F)F)cc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H27F7N4O5S/c1-3-47(44,45)14-13-40(25(42)16-19-6-11-24(31)23(15-19)30(35,36)37)18(2)27-39-26-22(5-4-12-38-26)28(43)41(27)20-7-9-21(10-8-20)46-17-29(32,33)34/h4-12,15,18H,3,13-14,16-17H2,1-2H3/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1P10 from human CXCR3 expressed in PBMC after 2 hrs in RPMI buffer by scintillation counting |
Bioorg Med Chem Lett 19: 5114-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.032
BindingDB Entry DOI: 10.7270/Q2FX79KZ |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358642

(CHEMBL1921891)Show SMILES CC[C@H]1CN(CCN1C1CCN(CC1)C(=O)c1ccc(Cl)cc1)c1ncc(nc1C)C(=O)NC1CC1 |r| Show InChI InChI=1S/C27H35ClN6O2/c1-3-22-17-33(25-18(2)30-24(16-29-25)26(35)31-21-8-9-21)14-15-34(22)23-10-12-32(13-11-23)27(36)19-4-6-20(28)7-5-19/h4-7,16,21-23H,3,8-15,17H2,1-2H3,(H,31,35)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM610414
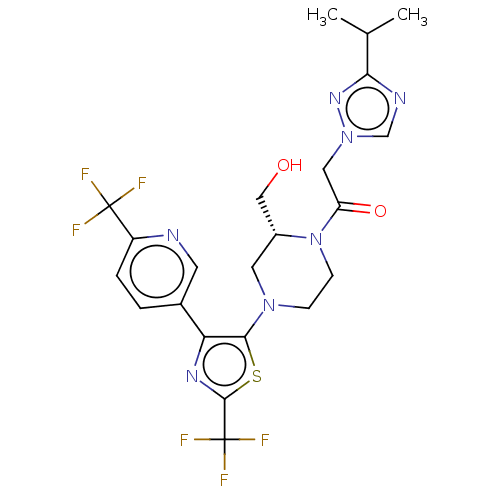
(1-{(R)-2-Hydroxymethyl-4-[2-trifluoromethyl-4-(6-t...)Show SMILES CC(C)c1ncn(CC(=O)N2CCN(C[C@@H]2CO)c2sc(nc2-c2ccc(nc2)C(F)(F)F)C(F)(F)F)n1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S46X2G |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50300902
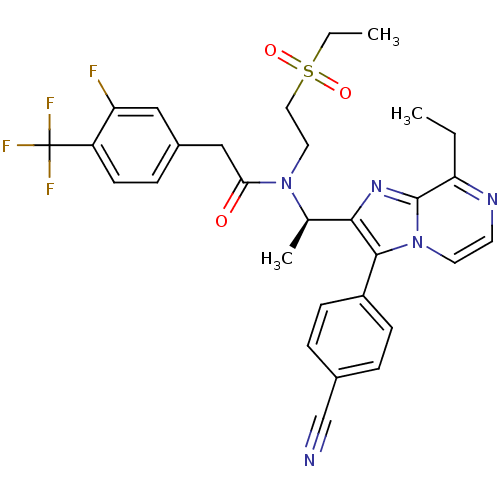
((R)-N-(1-(3-(4-cyanophenyl)-8-ethylimidazo[1,2-a]p...)Show SMILES CCc1nccn2c(c(nc12)[C@@H](C)N(CCS(=O)(=O)CC)C(=O)Cc1ccc(c(F)c1)C(F)(F)F)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C30H29F4N5O3S/c1-4-25-29-37-27(28(39(29)13-12-36-25)22-9-6-20(18-35)7-10-22)19(3)38(14-15-43(41,42)5-2)26(40)17-21-8-11-23(24(31)16-21)30(32,33)34/h6-13,16,19H,4-5,14-15,17H2,1-3H3/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM610432
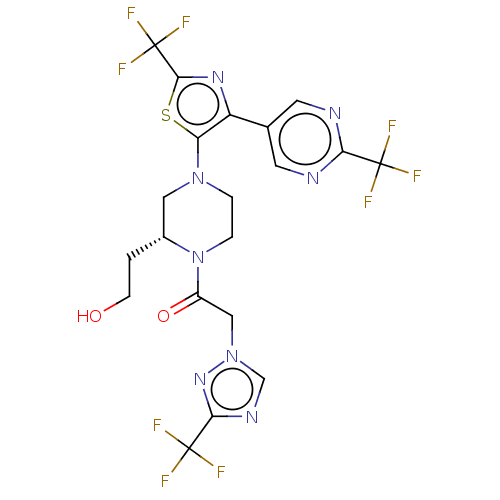
(1-{(R)-2-(2-Hydroxy-ethyl)-4-[2-trifluoromethyl-4-...)Show SMILES OCC[C@@H]1CN(CCN1C(=O)Cn1cnc(n1)C(F)(F)F)c1sc(nc1-c1cnc(nc1)C(F)(F)F)C(F)(F)F | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S46X2G |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50300906
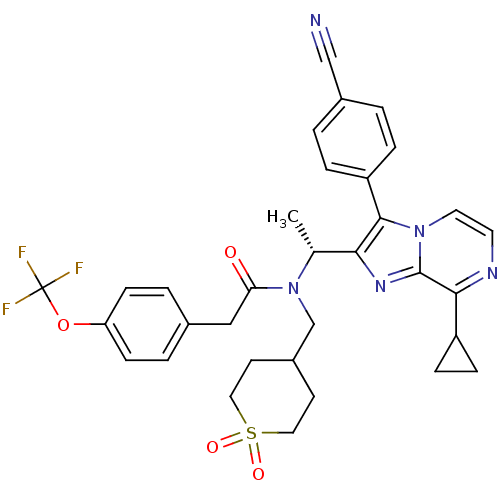
(CHEMBL576096 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...)Show SMILES C[C@@H](N(CC1CCS(=O)(=O)CC1)C(=O)Cc1ccc(OC(F)(F)F)cc1)c1nc2c(nccn2c1-c1ccc(cc1)C#N)C1CC1 |r| Show InChI InChI=1S/C33H32F3N5O4S/c1-21(29-31(26-6-2-23(19-37)3-7-26)40-15-14-38-30(25-8-9-25)32(40)39-29)41(20-24-12-16-46(43,44)17-13-24)28(42)18-22-4-10-27(11-5-22)45-33(34,35)36/h2-7,10-11,14-15,21,24-25H,8-9,12-13,16-18,20H2,1H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50300909
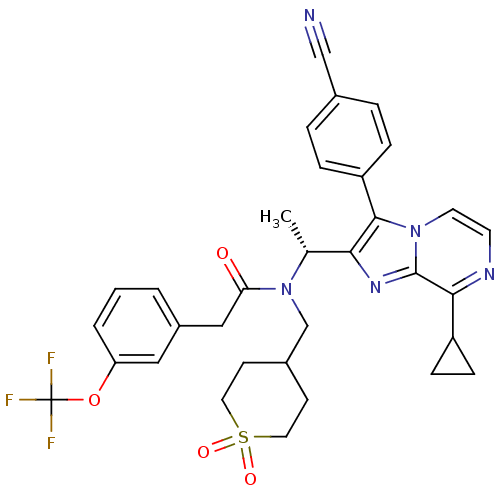
(CHEMBL576099 | N-{(R)-1-[3-(4-Cyano-phenyl)-8-cycl...)Show SMILES C[C@@H](N(CC1CCS(=O)(=O)CC1)C(=O)Cc1cccc(OC(F)(F)F)c1)c1nc2c(nccn2c1-c1ccc(cc1)C#N)C1CC1 |r| Show InChI InChI=1S/C33H32F3N5O4S/c1-21(29-31(26-7-5-22(19-37)6-8-26)40-14-13-38-30(25-9-10-25)32(40)39-29)41(20-23-11-15-46(43,44)16-12-23)28(42)18-24-3-2-4-27(17-24)45-33(34,35)36/h2-8,13-14,17,21,23,25H,9-12,15-16,18,20H2,1H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]IP10 from CXCR3 receptor expressed in PBMC |
Bioorg Med Chem Lett 19: 5200-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.021
BindingDB Entry DOI: 10.7270/Q2W95980 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM228188
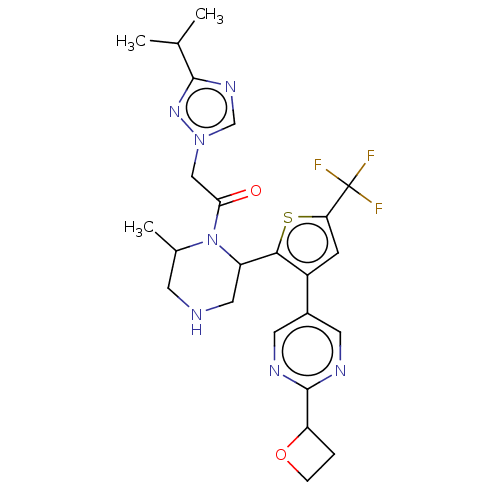
(1-{(R)-4-[4-(2-Cyclobutoxy-pyrimidin-5-yl)-2-trifl...)Show SMILES CC(C)c1ncn(CC(=O)N2C(C)CNCC2c2sc(cc2-c2cnc(nc2)C2CCO2)C(F)(F)F)n1 Show InChI InChI=1S/C24H28F3N7O2S/c1-13(2)22-31-12-33(32-22)11-20(35)34-14(3)7-28-10-17(34)21-16(6-19(37-21)24(25,26)27)15-8-29-23(30-9-15)18-4-5-36-18/h6,8-9,12-14,17-18,28H,4-5,7,10-11H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
IDORSIA PHARMACEUTICALS LTD.
US Patent
| Assay Description
The bioactivity of compounds is tested in a fluorometric imaging plate reader (FLIPR: Molecular Devices) using engineered CHO-K1 cells expressing the... |
US Patent US10047080 (2018)
BindingDB Entry DOI: 10.7270/Q2JQ130F |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50358629

(CHEMBL1921878)Show SMILES CC[C@H]1CN(CCN1C1CCN(CC1)C(=O)c1ccc(Cl)nc1N)c1ncc(nc1Cl)C(=O)NC1CC1 |r| Show InChI InChI=1S/C25H32Cl2N8O2/c1-2-16-14-34(23-21(27)31-19(13-29-23)24(36)30-15-3-4-15)11-12-35(16)17-7-9-33(10-8-17)25(37)18-5-6-20(26)32-22(18)28/h5-6,13,15-17H,2-4,7-12,14H2,1H3,(H2,28,32)(H,30,36)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in mouse BA/F3 cells |
Bioorg Med Chem Lett 21: 6982-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.120
BindingDB Entry DOI: 10.7270/Q2T72HVN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data