

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360985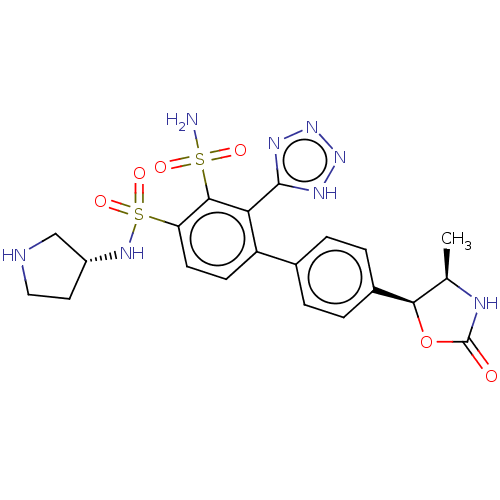 (4'-((4R,5S)-4-methyl-2- oxooxazolidin-5-yl)-N4-((R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360985 (4'-((4R,5S)-4-methyl-2- oxooxazolidin-5-yl)-N4-((R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368054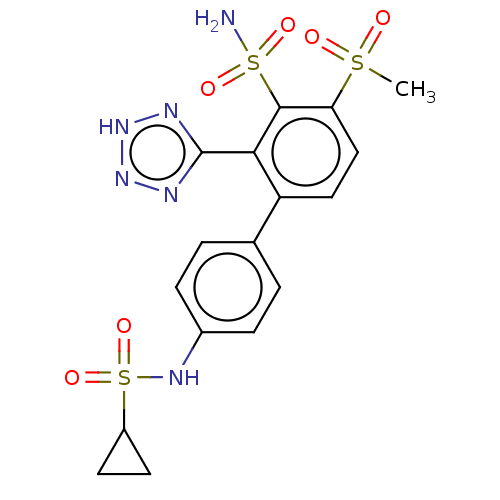 (4'- (Cyclopropanesulfonamido)- 4-(methylsulfonyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361227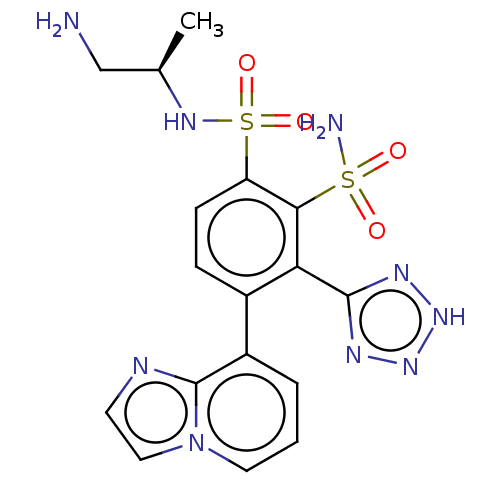 ((R)—N1-(1-aminopropan-2-yl)-4-(imidazo[1,2-a]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361227 ((R)—N1-(1-aminopropan-2-yl)-4-(imidazo[1,2-a]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361236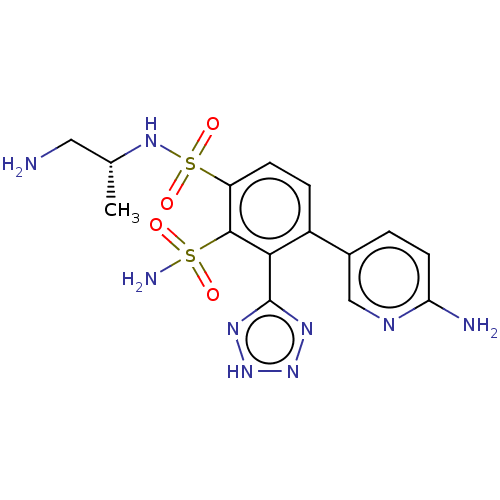 ((R)—N1-(1-aminopropan-2-yl)-4-(6-aminopyridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361236 ((R)—N1-(1-aminopropan-2-yl)-4-(6-aminopyridin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360850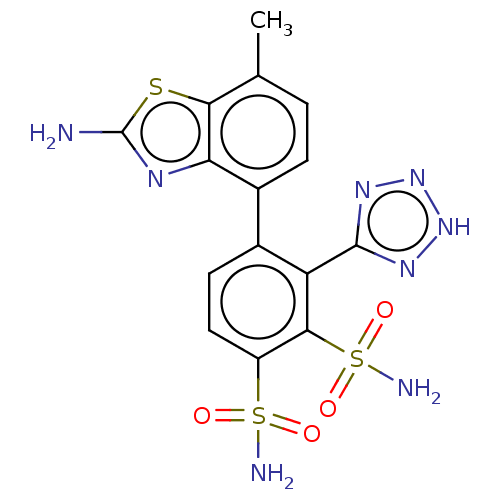 (4-(2-amino-7- methylbenzo[d]thiazol-4-yl)- 3-(2H-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360850 (4-(2-amino-7- methylbenzo[d]thiazol-4-yl)- 3-(2H-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360980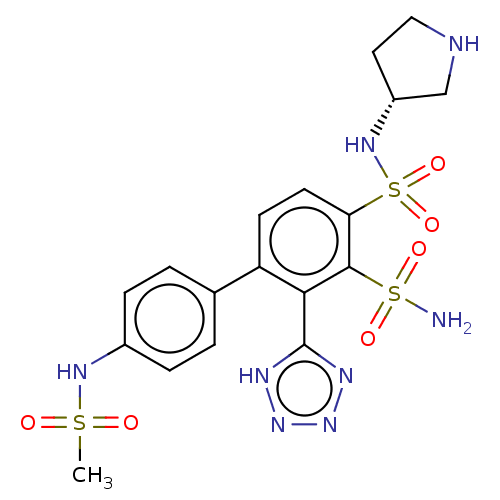 ((R)-4'-(methylsulfonamido)-N4- (pyrrolidin-3-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361209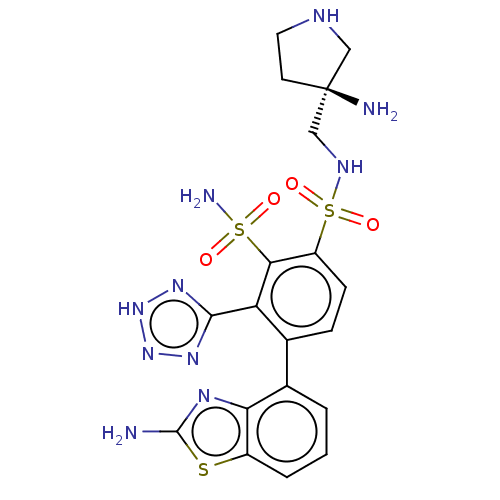 ((S)-4-(2-aminobenzo[d]thiazol-4-yl)-N1-((3-aminopy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361209 ((S)-4-(2-aminobenzo[d]thiazol-4-yl)-N1-((3-aminopy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360980 ((R)-4'-(methylsulfonamido)-N4- (pyrrolidin-3-yl)-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361341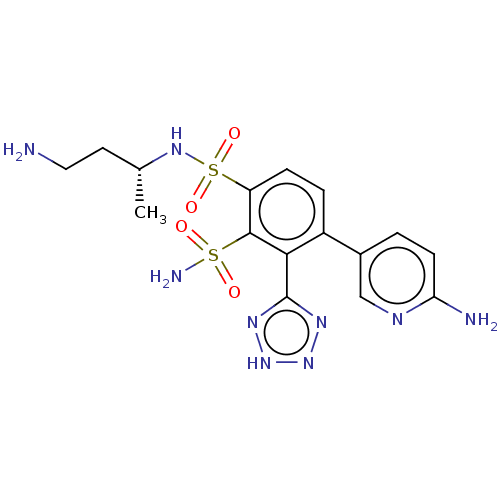 ((R)-N1-(4-aminobutan-2- yl)-4-(6-aminopyridin-3- y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361341 ((R)-N1-(4-aminobutan-2- yl)-4-(6-aminopyridin-3- y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368000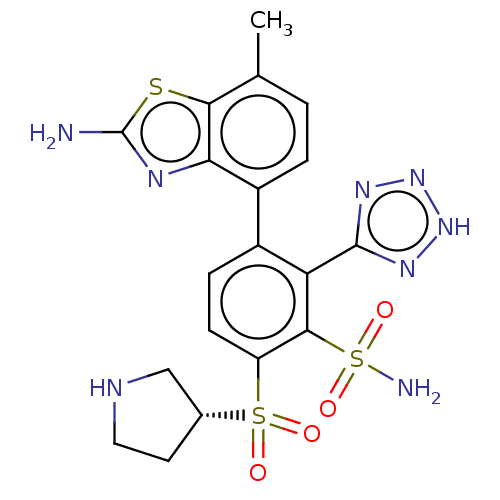 (3-(2-amino-7- methylbenzo[d]thiazol-4-yl)-6- ((R)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361210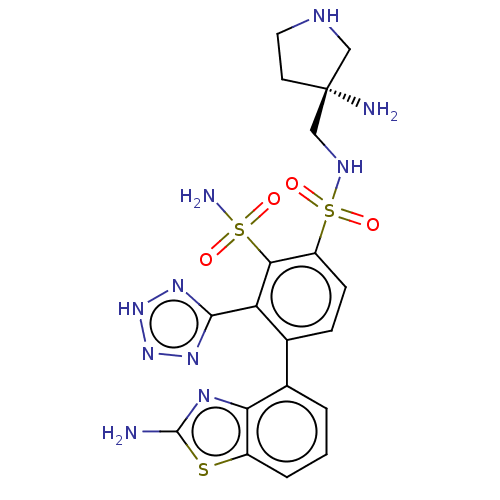 ((R)-4-(2-aminobenzo[d]thiazol-4-yl)-N1-((3-aminopy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361210 ((R)-4-(2-aminobenzo[d]thiazol-4-yl)-N1-((3-aminopy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM367893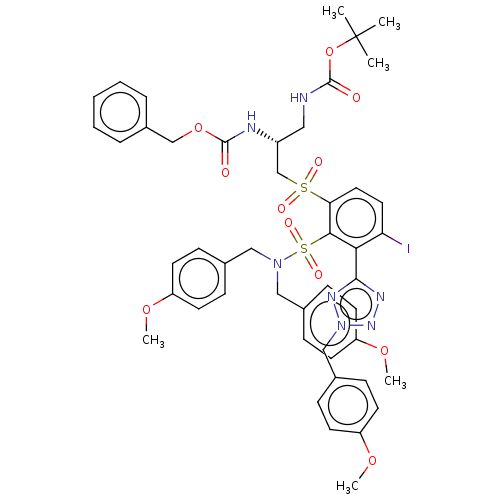 ((R)-benzyl tert-butyl (3-((2- (N,N-bis(4- methoxyb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368120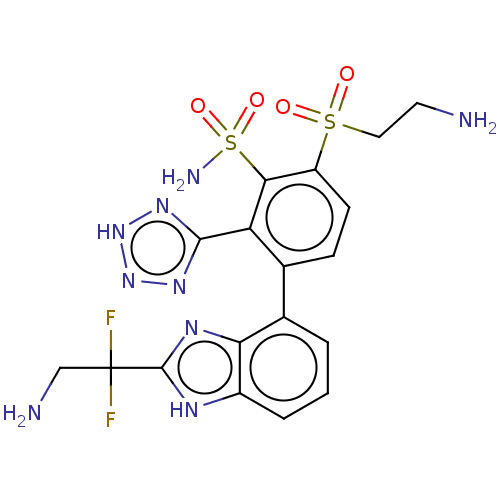 (3-(2-(2-amino-1,1- difluoroethyl)-1H- benzo[d]imid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368052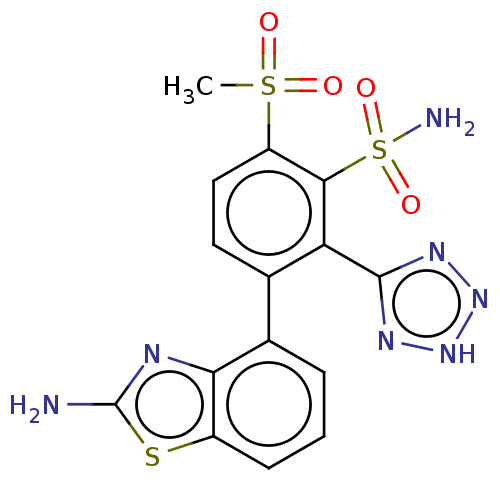 (3-(2-aminobenzo[d]thiazol- 4-yl)-6-(methylsulfonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368006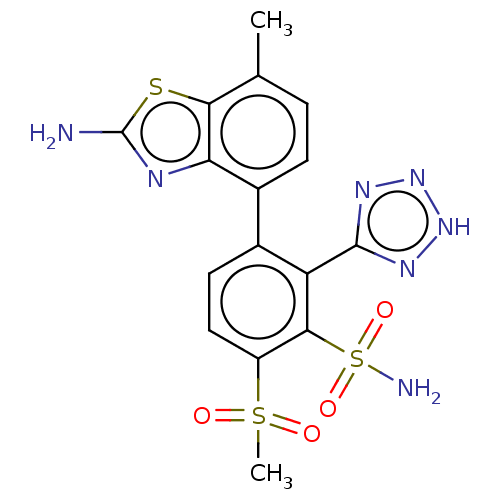 (3-(2-amino-7- methylbenzo[d]thiazol-4-yl)-6- (meth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM367903 (6-(2-aminoethylsulfonyl)-3-(2-(methylamino)benzo[d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361042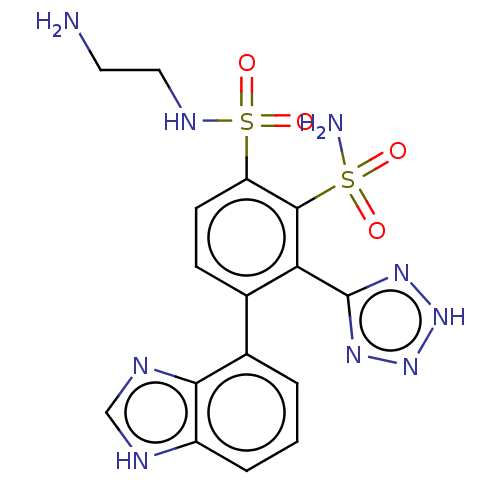 (N1-(2-aminoethyl)-4-(1H-benzo[d]imidazol-4-yl)-3-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361042 (N1-(2-aminoethyl)-4-(1H-benzo[d]imidazol-4-yl)-3-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361273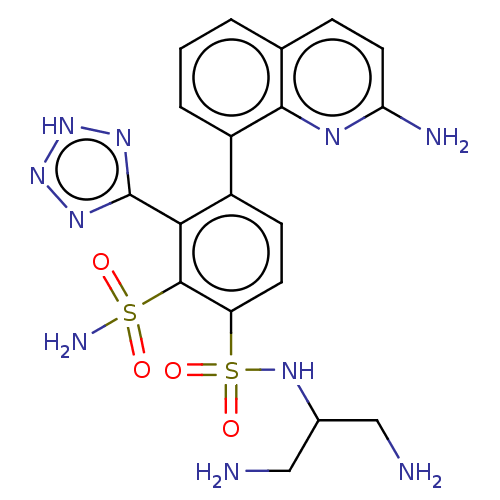 (US10221163, Example 430 | US10544130, Example 430) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361273 (US10221163, Example 430 | US10544130, Example 430) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.102 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361279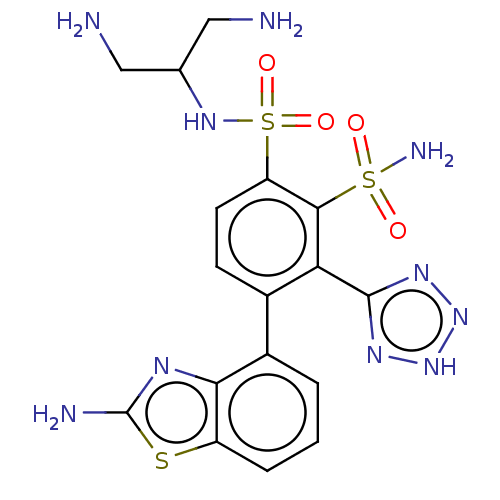 (US10221163, Example 436 | US10544130, Example 436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361279 (US10221163, Example 436 | US10544130, Example 436) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368058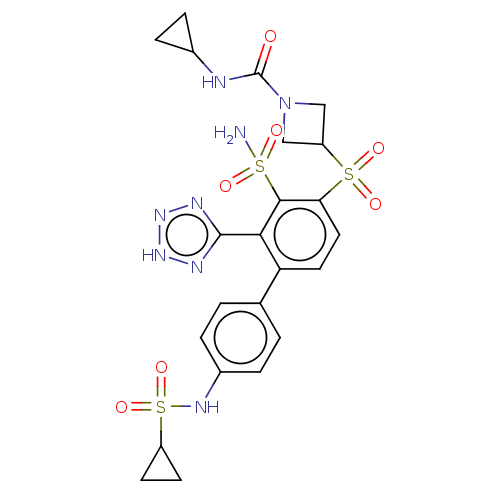 (3-((4′-(Cyclopropanesulfonamido)-3-sulfamoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368049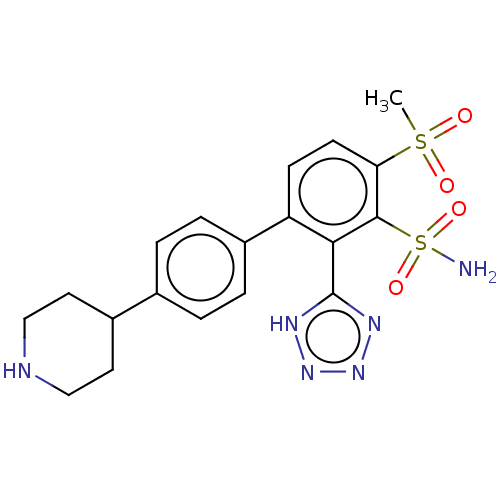 (4-(methylsulfonyl)-4′-(piperidin-4-yl)-2-(1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.103 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM360855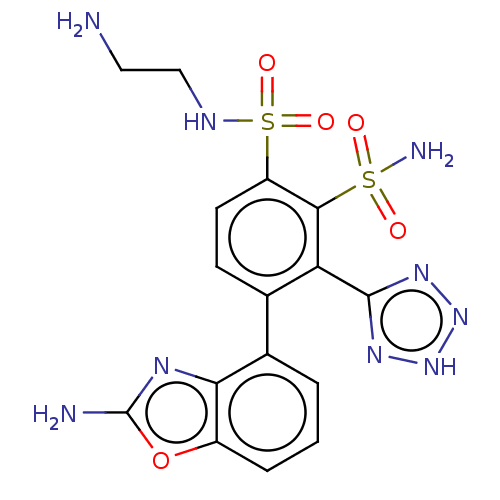 (4-(2-aminobenzo[d]oxazol- 4-yl)-N1-(2-aminoethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.105 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM367839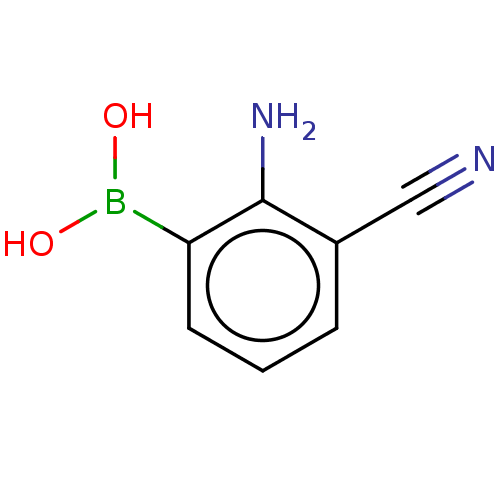 ((2-amino-3- cyanophenyl) boronic acid | US10227331...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.105 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361044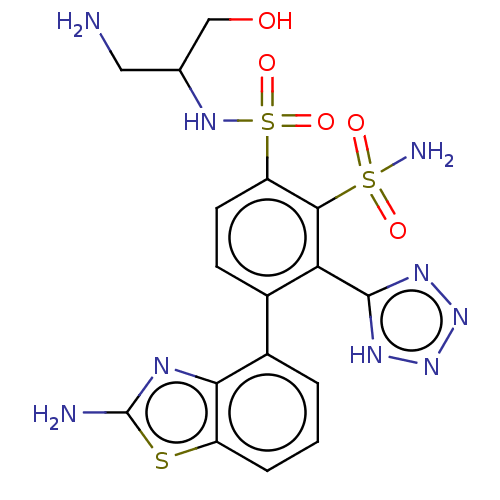 ((S)—N1-(1-amino-3-hydroxypropan-2-yl)-4-(2-am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.109 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361044 ((S)—N1-(1-amino-3-hydroxypropan-2-yl)-4-(2-am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.109 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368001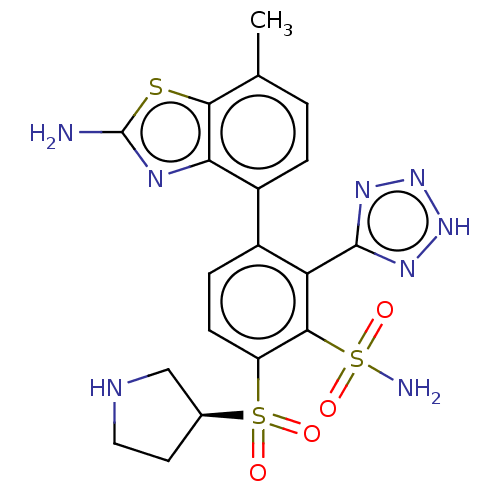 (3-(2-amino-7- methylbenzo[d]thiazol-4-yl)-6- ((S)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.109 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361068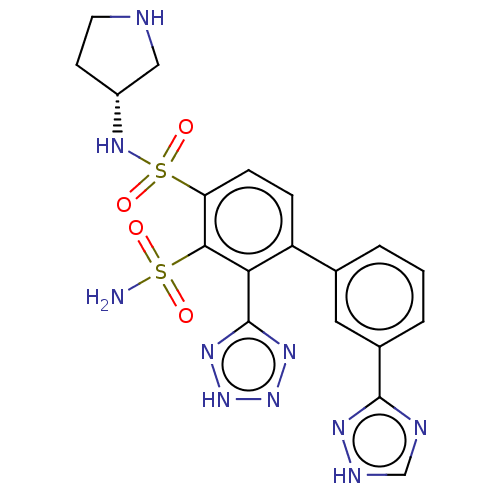 ((R)-N4-(pyrrolidin-3-yl)-2-(2H- tetrazol-5-yl)-3'-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361068 ((R)-N4-(pyrrolidin-3-yl)-2-(2H- tetrazol-5-yl)-3'-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361314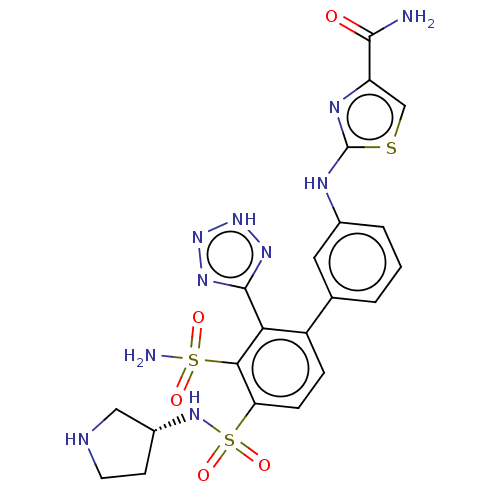 ((R)-2-((4′-(N-(pyrrolidin-3-yl)sulfamoyl)-3&...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.111 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361314 ((R)-2-((4′-(N-(pyrrolidin-3-yl)sulfamoyl)-3&...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.111 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361080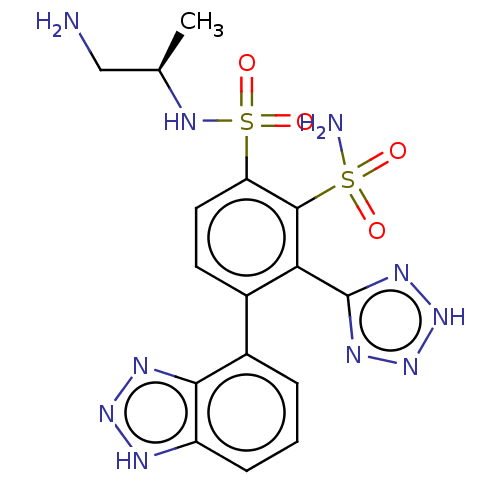 (US10221163, Example 237 | US10544130, Example 237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361080 (US10221163, Example 237 | US10544130, Example 237) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361129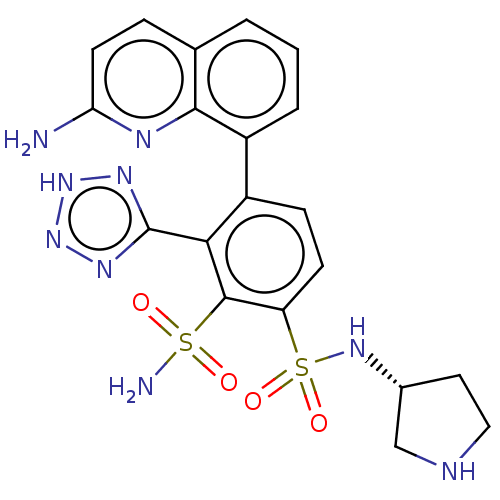 ((R)-4-(2-aminoquinolin- 8-yl)-N1-(pyrrolidin-3- yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361129 ((R)-4-(2-aminoquinolin- 8-yl)-N1-(pyrrolidin-3- yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM367851 (2-amino-4-(4-(azetidin-3-ylsulfonyl)-3-sulfamoyl-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368094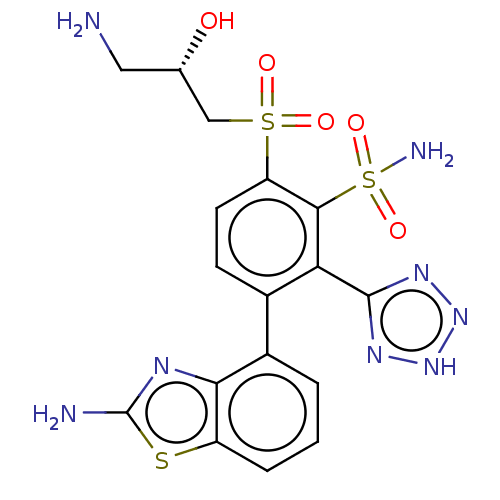 (6-((S)-3-amino-2-hydroxypropylsulfonyl)-3-(2-amino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM368058 (3-((4′-(Cyclopropanesulfonamido)-3-sulfamoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361172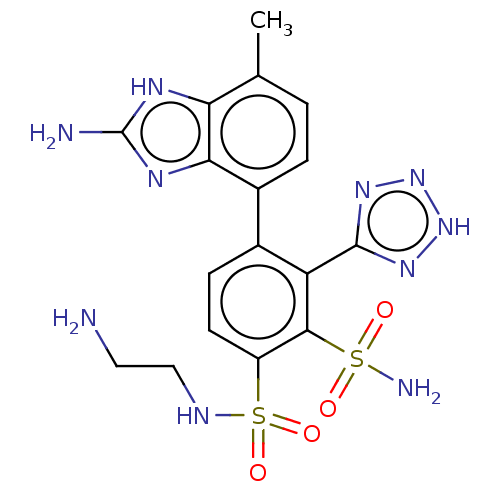 (4-(2-Amino-7-methyl-1H-benzo[d]imidazol-4-yl)-N1-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10221163 (2019) BindingDB Entry DOI: 10.7270/Q2RX9FB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM361172 (4-(2-Amino-7-methyl-1H-benzo[d]imidazol-4-yl)-N1-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.128 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | US Patent US10544130 (2020) BindingDB Entry DOI: 10.7270/Q2C82CQH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Serratia marcescens) | BDBM367884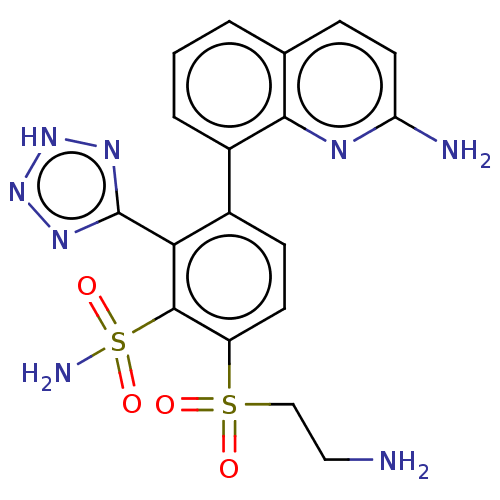 (6-(2-aminoethylsulfonyl)-3-(2- aminoquinolin-8-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The Class B enzyme activities were measured in the presence of the test inhibitor in a fluorescence assay against a commercially available substrate ... | J Med Chem 51: 1231-41 (2008) BindingDB Entry DOI: 10.7270/Q2Z60RCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2266 total ) | Next | Last >> |