

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476758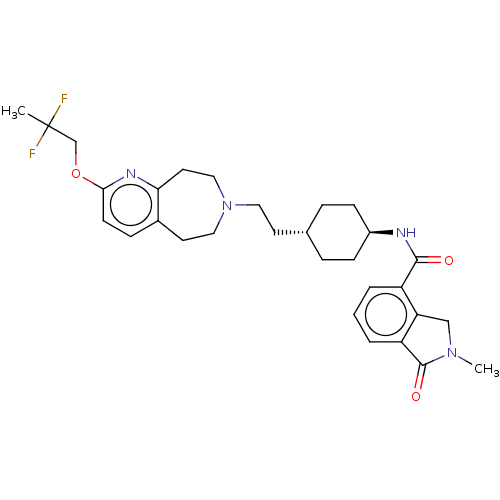 (US10870660, Compound III-024 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476918 (US10870660, Compound II-057) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593894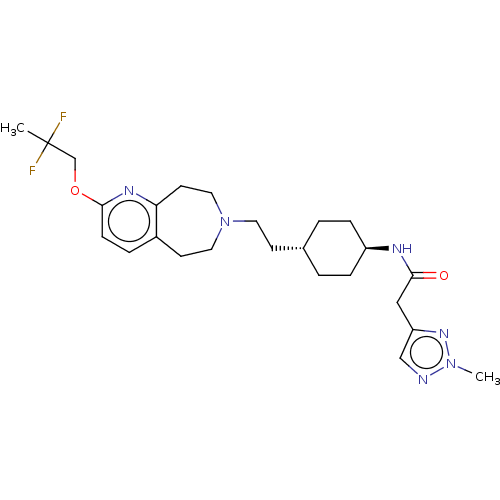 (US11578084, Compound I'-42) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290221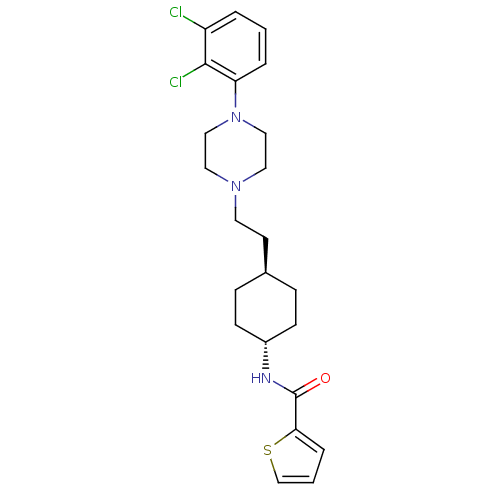 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063292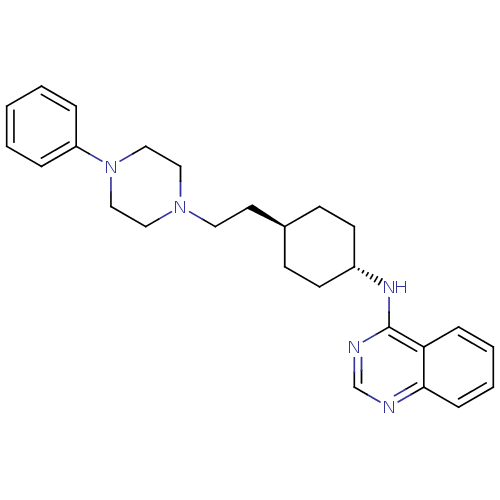 (CHEMBL349426 | {4-[2-(4-Phenyl-piperazin-1-yl)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity determined by measuring displacement of [3H]-spiperone from cloned Human Dopamine receptor D3 in CHO-K1 cells | J Med Chem 41: 760-71 (1998) Article DOI: 10.1021/jm9707378 BindingDB Entry DOI: 10.7270/Q20G3J97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476915 (US10870660, Compound II-047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593791 (US11578084, Compound I-074) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476764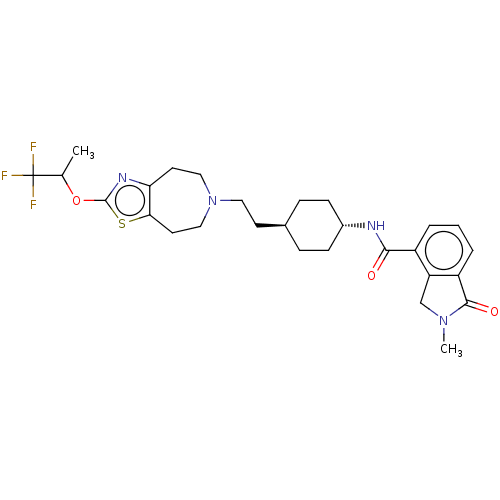 (US10870660, Compound III-064 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50341508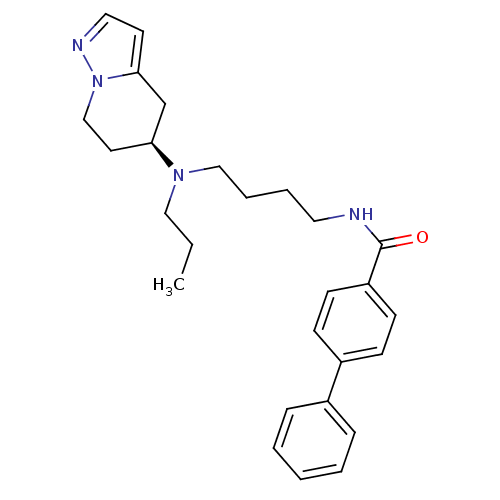 ((S)-N-(4-(4-Phenylbenzoylamino)butyl)-N-propyl-5-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Friedrich Alexander University Curated by ChEMBL | Assay Description Displacement of [3H]7-OH-DPAT from human dopamine D3 receptor expressed in CHO cells after 60 mins | J Med Chem 54: 2477-91 (2011) Article DOI: 10.1021/jm101639t BindingDB Entry DOI: 10.7270/Q2V1253W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593929 (US11578084, Compound I-157) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207116 (CHEMBL3905247 | US9550741, I-4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593940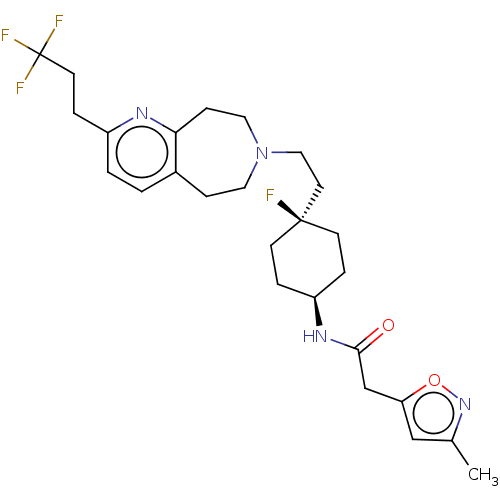 (US11578084, Compound III-3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593840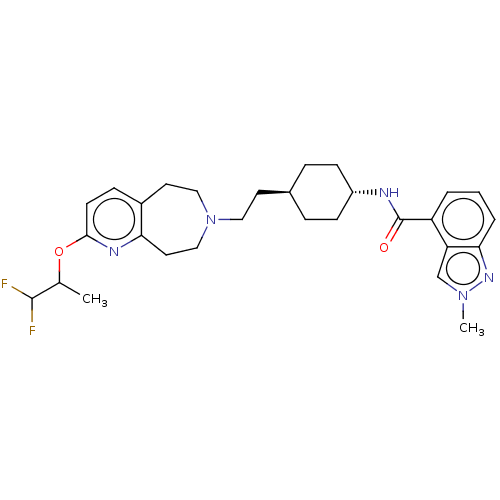 (US11578084, Compound I-123 | US11578084, Compound ...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476836 (US10870660, Compound III-581 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593735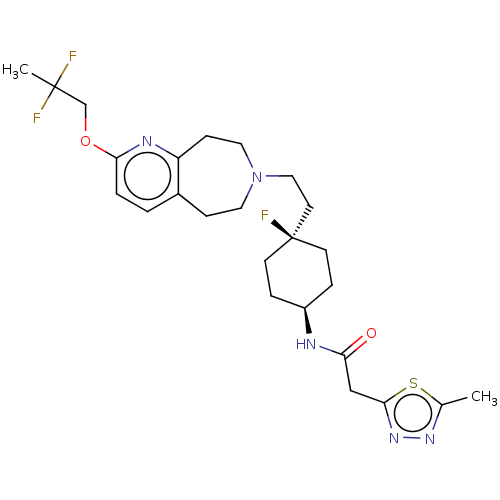 (US11578084, Compound I-018) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593733 (US11578084, Compound I-016) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Collegium Medicum Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human dopamine D3 receptor expressed in CHO cells | Eur J Med Chem 92: 221-35 (2015) Article DOI: 10.1016/j.ejmech.2014.12.045 BindingDB Entry DOI: 10.7270/Q2Q241XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Adamed Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]methylspiperone from human recombinant D3 receptor expressed in CHO cells | J Med Chem 57: 4543-57 (2014) Article DOI: 10.1021/jm401895u BindingDB Entry DOI: 10.7270/Q2N29ZHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207143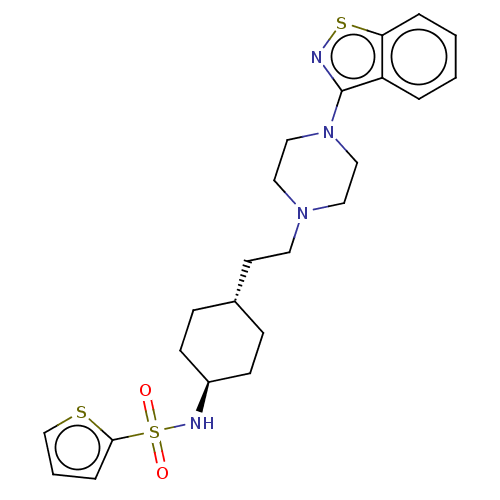 (CHEMBL3966842 | US9550741, II-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207162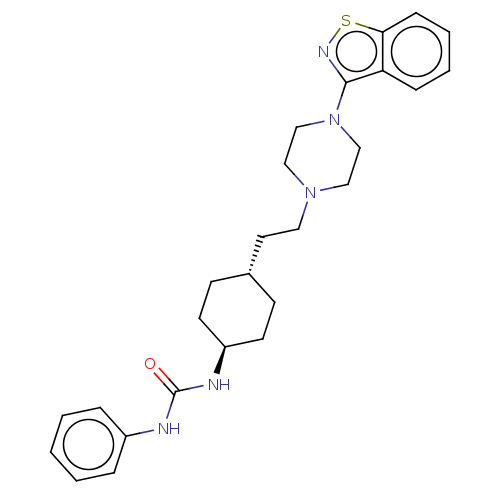 (CHEMBL3918755 | US9550741, IV-1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593890 (US11578084, Compound I'-38) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593802 (US11578084, Compound I-085) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207155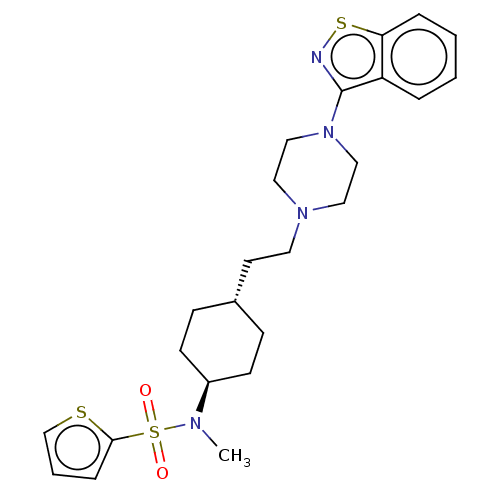 (CHEMBL3895540) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207141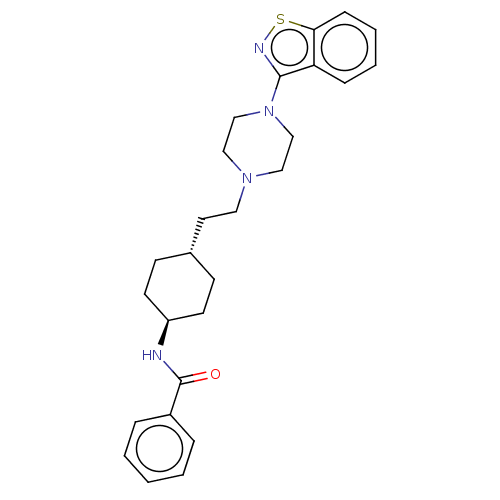 (CHEMBL3920252) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593765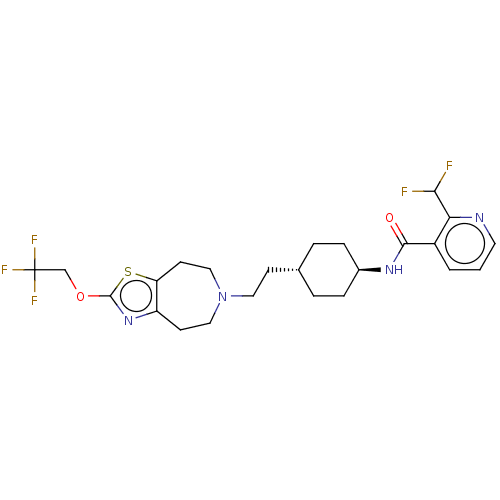 (US11578084, Compound I-048) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476923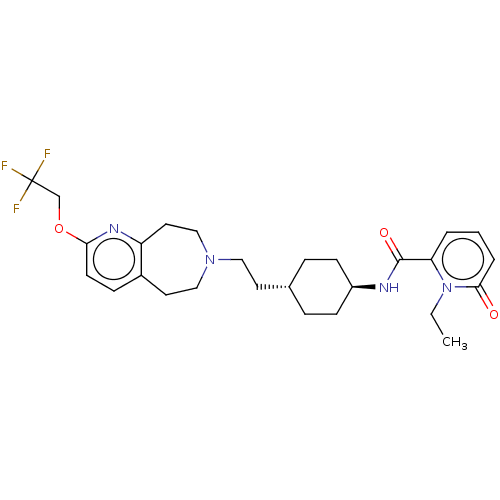 (US10870660, Compound II-081 | US11345716, Compound...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476834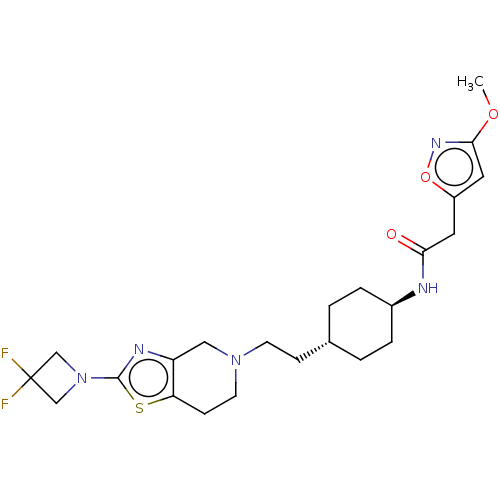 (US10870660, Compound III-578 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207148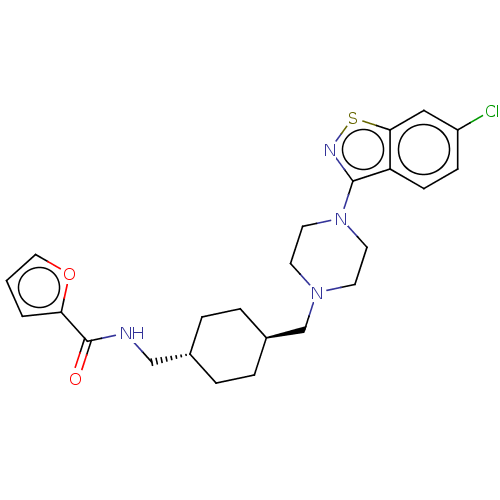 (CHEMBL3932186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476858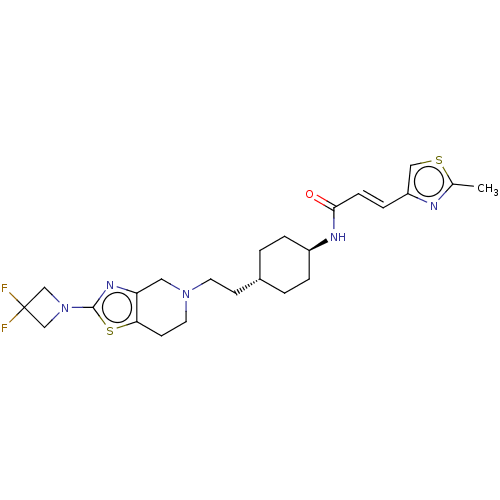 (US10870660, Compound III-706 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476924 (US10870660, Compound II-084 | US11345716, Compound...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207154 (CHEMBL3902496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207113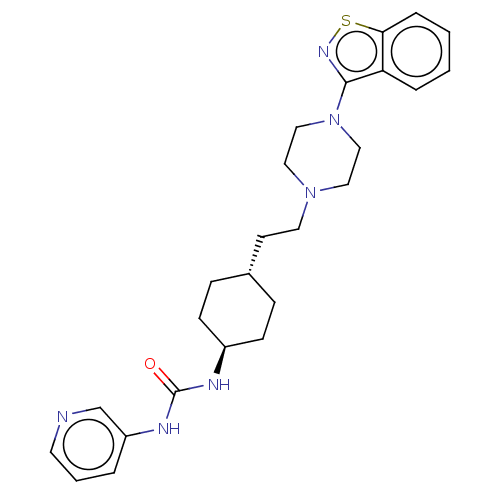 (CHEMBL3896937 | US9550741, IV-3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207094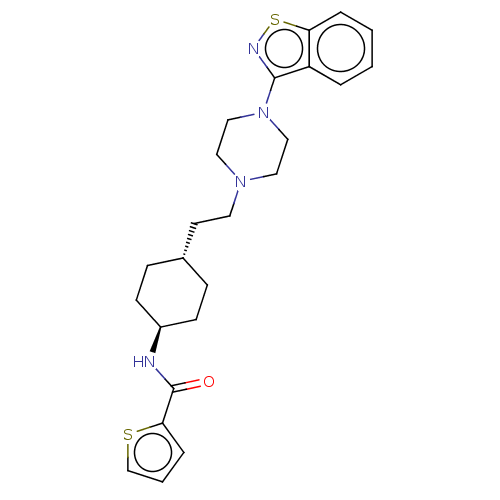 (CHEMBL3976282 | US9550741, I-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476773 (US10870660, Compound III-138 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207158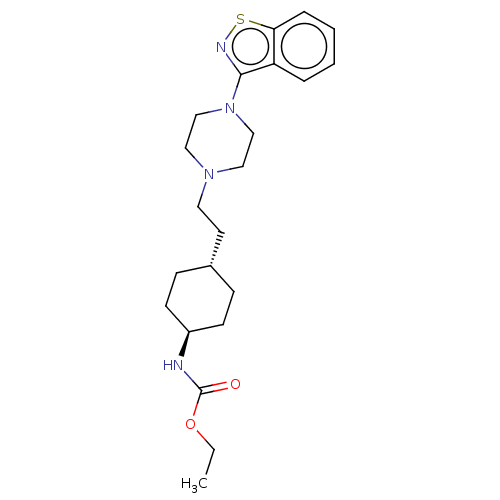 (CHEMBL3982486 | US9550741, III-2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476914 (US10870660, Compound II-041) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593764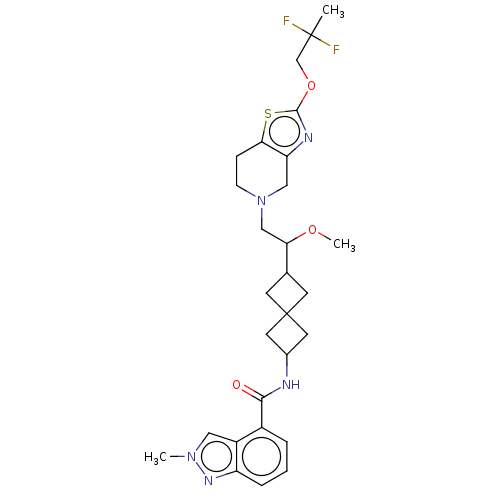 (US11578084, Compound I-047) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207150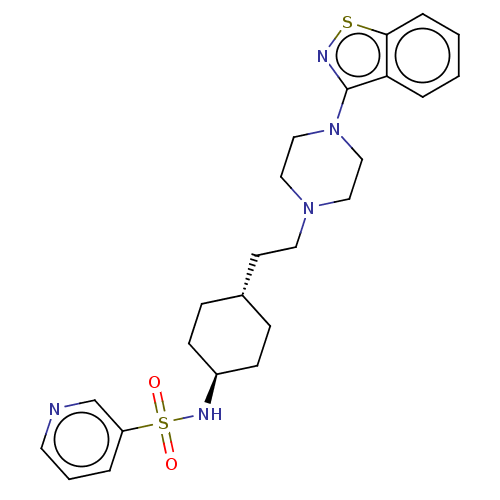 (CHEMBL3933256) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476925 (US10870660, Compound II-087) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM571885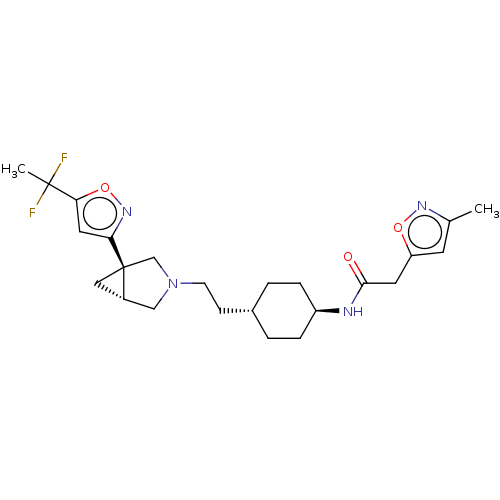 (US11447484, Compound I-042) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl2.6H2O (20909-55, Nac... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RN3C31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50207115 (CHEMBL3948056) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University Curated by ChEMBL | Assay Description Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cell membranes after 60 mins by scintillation counting metho... | Eur J Med Chem 123: 332-353 (2016) Article DOI: 10.1016/j.ejmech.2016.07.038 BindingDB Entry DOI: 10.7270/Q2GH9KXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593734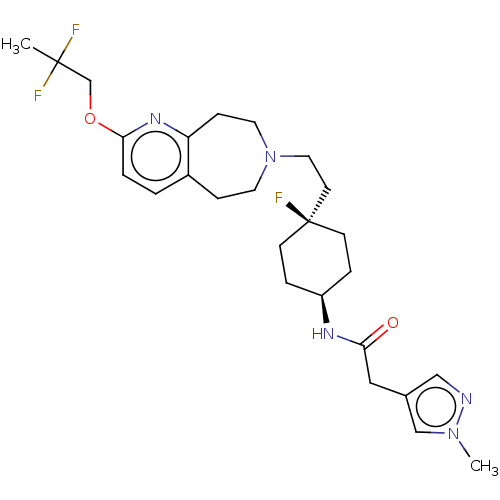 (US11578084, Compound I-017) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476759 (US10870660, Compound III-027 | US11345716, Compoun...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM476866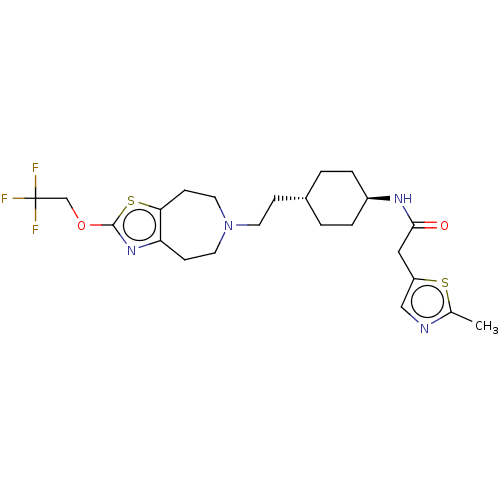 (US10870660, Compound II-053 | US11345716, Compound...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description 225 nL of the solutions of the non-specific ligand or the compounds of the present invention at each concentration (in case of vehicle, final concent... | US Patent US10870660 (2020) BindingDB Entry DOI: 10.7270/Q2M61PBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192240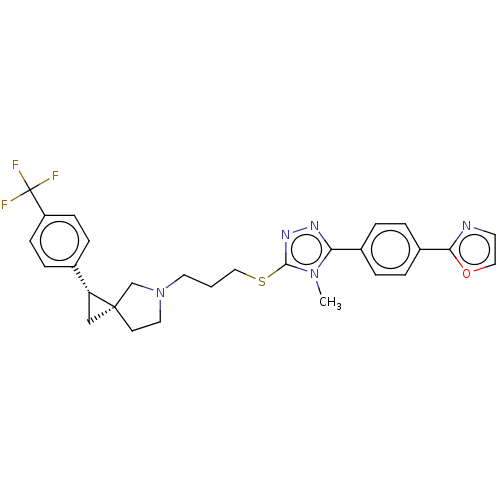 (CHEMBL3941818 | US10239870, Example 278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Displacement of [3H]-spiperone from human dopamine D3 receptor expressed in CHO-K1 cell membranes after 90 mins by liquid scintillation counting | J Med Chem 59: 8549-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00972 BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593796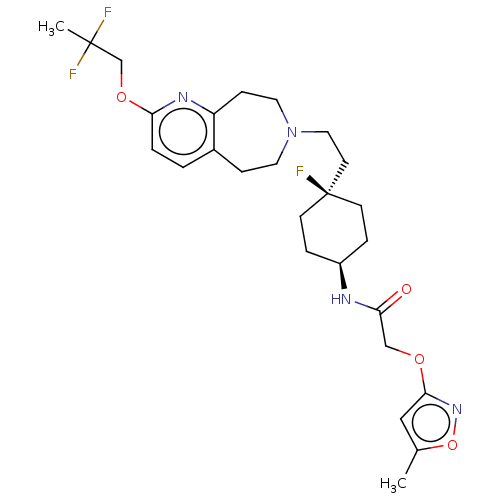 (US11578084, Compound I-079) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192240 (CHEMBL3941818 | US10239870, Example 278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding... | J Med Chem 59: 8549-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00972 BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50192240 (CHEMBL3941818 | US10239870, Example 278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aptuit s.r.l. Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D3 receptor expressed in CHO cell membranes after 90 mins in presence of quinelorane by [35S]-GTPgammaS binding... | J Med Chem 59: 8549-76 (2016) Article DOI: 10.1021/acs.jmedchem.6b00972 BindingDB Entry DOI: 10.7270/Q2SQ9298 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593860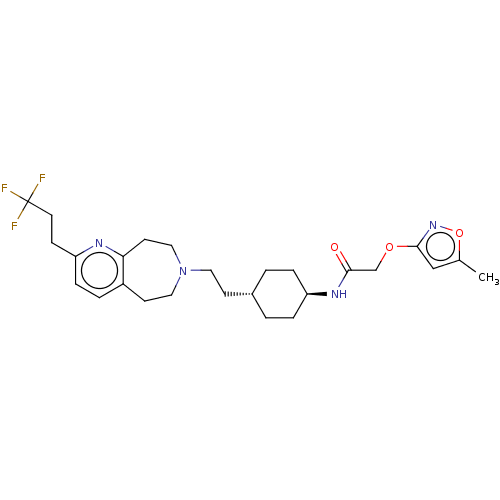 (US11578084, Compound I'-8) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens) | BDBM593888 (US11578084, Compound I'-36) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Buffer solution: 50 mM Tris-HCl (35409-45, Nacalai Tesque) (pH 7.4) containing 120 mM NaCl (31320-05, Nacalai Tesque), 1 mM MgCl.6HO (20909-55, Nacal... | Citation and Details BindingDB Entry DOI: 10.7270/Q2CF9V1Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7365 total ) | Next | Last >> |