 Found 32 hits of ic50 for UniProtKB: P30939
Found 32 hits of ic50 for UniProtKB: P30939 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573968
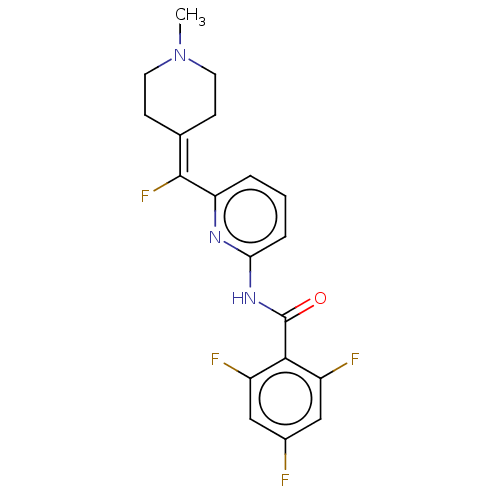
(CHEMBL4851044)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2c(F)cc(F)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT1F receptor |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573966
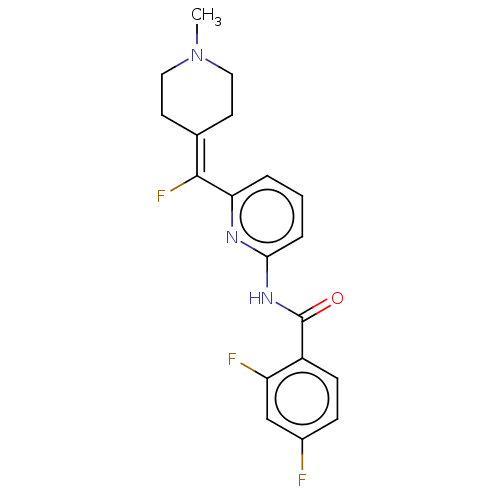
(CHEMBL4847626)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2ccc(F)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573964
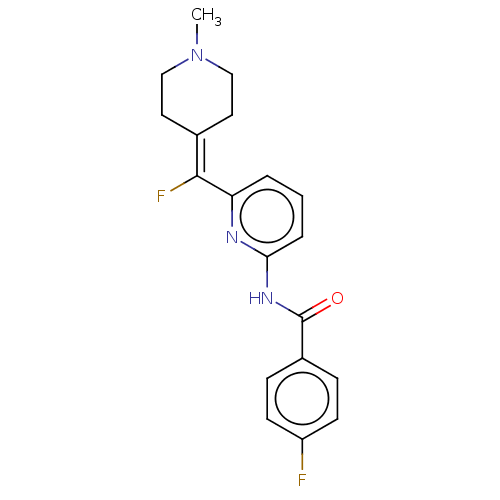
(CHEMBL4846825)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2ccc(F)cc2)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573968

(CHEMBL4851044)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2c(F)cc(F)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573967

(CHEMBL4865700)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2ccc(Cl)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573969

(CHEMBL4860576)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2ccc(F)cn2)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573965

(CHEMBL4862526)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2ccc(Cl)cc2)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573975

(COL-144 | LY573144 | Lasmiditan)Show SMILES CN1CCC(CC1)C(=O)c1cccc(NC(=O)c2c(F)cc(F)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573970
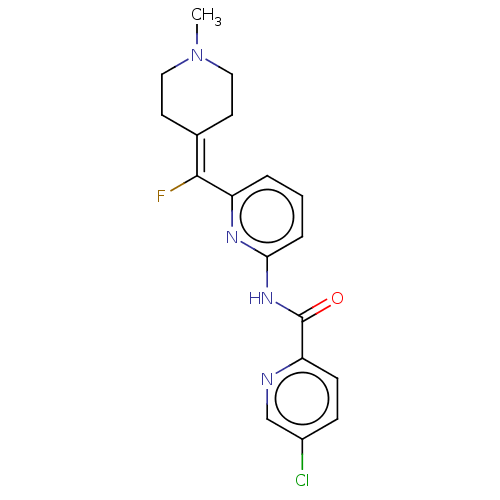
(CHEMBL4852743)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2ccc(Cl)cn2)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573971

(CHEMBL4874958)Show SMILES [#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2c(F)cc(F)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50473503

(CHEMBL2110300)Show SMILES CN(C)[C@H]1C[C@@H](C1)c1c[nH]c2ccc(CC3COC(=O)N3)cc12 |wU:3.2,wD:5.7,(12.22,-9.08,;13.25,-7.93,;14.76,-8.24,;12.76,-6.47,;11.38,-5.77,;12.08,-4.41,;13.46,-5.09,;11.59,-2.95,;12.5,-1.69,;11.59,-.45,;10.13,-.94,;8.79,-.17,;7.46,-.94,;7.46,-2.48,;6.12,-3.25,;6.12,-4.79,;4.88,-5.7,;5.36,-7.16,;6.9,-7.17,;7.98,-8.25,;7.37,-5.7,;8.79,-3.25,;10.13,-2.48,)| Show InChI InChI=1S/C18H23N3O2/c1-21(2)14-7-12(8-14)16-9-19-17-4-3-11(6-15(16)17)5-13-10-23-18(22)20-13/h3-4,6,9,12-14,19H,5,7-8,10H2,1-2H3,(H,20,22)/t12-,13?,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 363 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome
Curated by ChEMBL
| Assay Description
Binding affinity towards human recombinant 5-hydroxytryptamine 1F receptor |
J Med Chem 44: 681-93 (2001)
Article DOI: 10.1021/jm000956k
BindingDB Entry DOI: 10.7270/Q2PC354M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573972

(CHEMBL4872571)Show SMILES F\[#6](=[#6]-1\[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6]-1-[#6]-[#6]-1)-c1cccc(-[#7]-[#6](=O)-c2c(F)cc(F)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 571 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573973

(CHEMBL4871295)Show SMILES F[#6](F)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\F)-c1cccc(-[#7]-[#6](=O)-c2c(F)cc(F)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 813 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50005835

((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...)Show InChI InChI=1S/C14H21N3O2S/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3/h4-5,8-9,15-16H,6-7,10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for the affinity at 5-hydroxytryptamine 1F receptor |
J Med Chem 40: 3501-3 (1997)
Article DOI: 10.1021/jm9704560
BindingDB Entry DOI: 10.7270/Q2DZ07D8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50224302
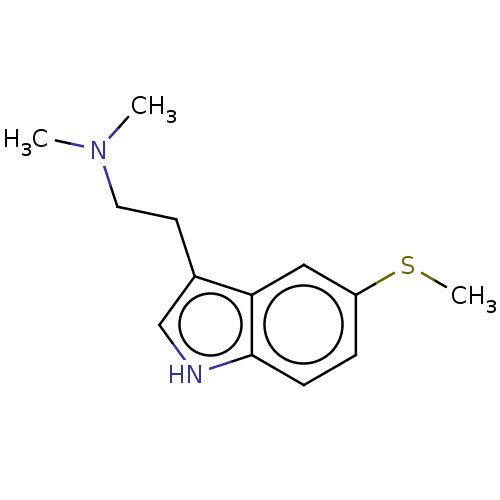
(CHEMBL31783)Show InChI InChI=1S/C13H18N2S/c1-15(2)7-6-10-9-14-13-5-4-11(16-3)8-12(10)13/h4-5,8-9,14H,6-7H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]5-HT binding from 5-hydroxytryptamine receptor site using 1 uM LSD as masking ligand |
J Med Chem 25: 908-13 (1982)
BindingDB Entry DOI: 10.7270/Q2H70J1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50224300
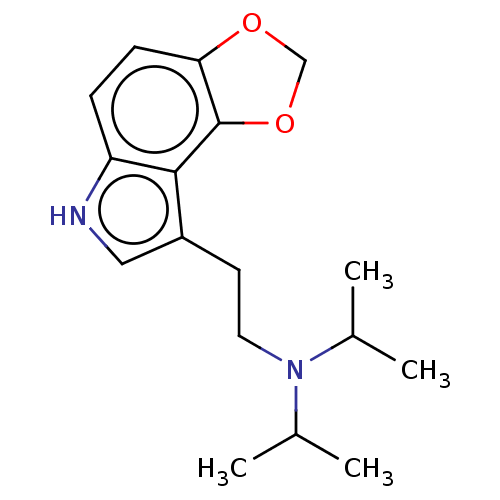
(CHEMBL352315)Show InChI InChI=1S/C17H24N2O2/c1-11(2)19(12(3)4)8-7-13-9-18-14-5-6-15-17(16(13)14)21-10-20-15/h5-6,9,11-12,18H,7-8,10H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]5-HT binding to 5-hydroxytryptamine receptor site using 1 uM LSD as masking ligand |
J Med Chem 25: 908-13 (1982)
BindingDB Entry DOI: 10.7270/Q2H70J1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50060437
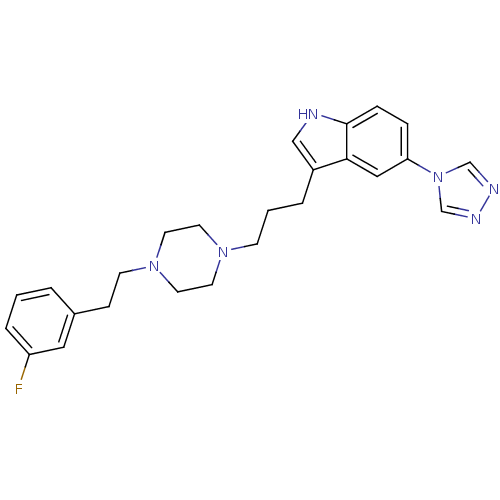
(3-(3-{4-[2-(3-Fluoro-phenyl)-ethyl]-piperazin-1-yl...)Show SMILES Fc1cccc(CCN2CCN(CCCc3c[nH]c4ccc(cc34)-n3cnnc3)CC2)c1 Show InChI InChI=1S/C25H29FN6/c26-22-5-1-3-20(15-22)8-10-31-13-11-30(12-14-31)9-2-4-21-17-27-25-7-6-23(16-24(21)25)32-18-28-29-19-32/h1,3,5-7,15-19,27H,2,4,8-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for the affinity at 5-hydroxytryptamine 1F receptor |
J Med Chem 40: 3501-3 (1997)
Article DOI: 10.1021/jm9704560
BindingDB Entry DOI: 10.7270/Q2DZ07D8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50060429
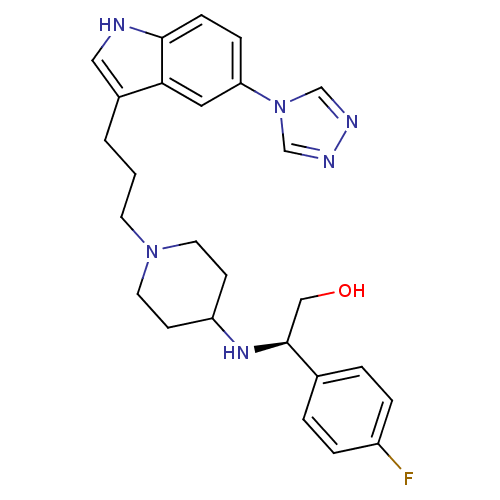
((R)-2-(4-Fluoro-phenyl)-2-{1-[3-(5-[1,2,4]triazol-...)Show SMILES OC[C@H](NC1CCN(CCCc2c[nH]c3ccc(cc23)-n2cnnc2)CC1)c1ccc(F)cc1 Show InChI InChI=1S/C26H31FN6O/c27-21-5-3-19(4-6-21)26(16-34)31-22-9-12-32(13-10-22)11-1-2-20-15-28-25-8-7-23(14-24(20)25)33-17-29-30-18-33/h3-8,14-15,17-18,22,26,28,31,34H,1-2,9-13,16H2/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for the displacement of [3H]-5-HT binding to cloned human 5-hydroxytryptamine 1F receptor stably expressed in CHO cells |
J Med Chem 42: 4981-5001 (2000)
BindingDB Entry DOI: 10.7270/Q20P0Z77 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1F
(Homo sapiens (Human)) | BDBM50573974

(CHEMBL4861949)Show SMILES F\[#6](=[#6]-1\[#6]-[#6]-[#7](-[#6]-[#6]-1)C(F)(F)F)-c1cccc(-[#7]-[#6](=O)-c2c(F)cc(F)cc2F)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at 5HT1F receptor (unknown origin) expressed in HEK293 cells measured after 60 mins by Fluo-4AM dye based FLIPR assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113782
BindingDB Entry DOI: 10.7270/Q2TQ65CK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50224301

(CHEMBL417480)Show InChI InChI=1S/C13H18N2S/c1-15(2)7-6-10-9-14-13-8-11(16-3)4-5-12(10)13/h4-5,8-9,14H,6-7H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]5-HT binding to 5-hydroxytryptamine receptor site using 1 uM LSD as masking ligand |
J Med Chem 25: 908-13 (1982)
BindingDB Entry DOI: 10.7270/Q2H70J1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50224303
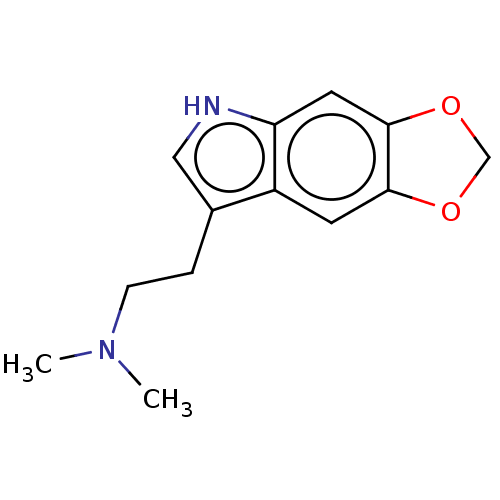
(CHEMBL283686)Show InChI InChI=1S/C13H16N2O2/c1-15(2)4-3-9-7-14-11-6-13-12(5-10(9)11)16-8-17-13/h5-7,14H,3-4,8H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]5-HT binding to 5-hydroxytryptamine receptor site using 1 uM LSD as masking ligand |
J Med Chem 25: 908-13 (1982)
BindingDB Entry DOI: 10.7270/Q2H70J1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50224305
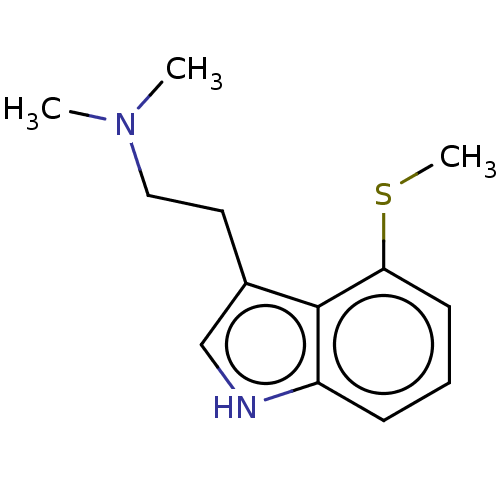
(CHEMBL285355)Show InChI InChI=1S/C13H18N2S/c1-15(2)8-7-10-9-14-11-5-4-6-12(16-3)13(10)11/h4-6,9,14H,7-8H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.51E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]5-HT binding to 5-hydroxytryptamine receptor site using 1 uM LSD as masking ligand |
J Med Chem 25: 908-13 (1982)
BindingDB Entry DOI: 10.7270/Q2H70J1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022536
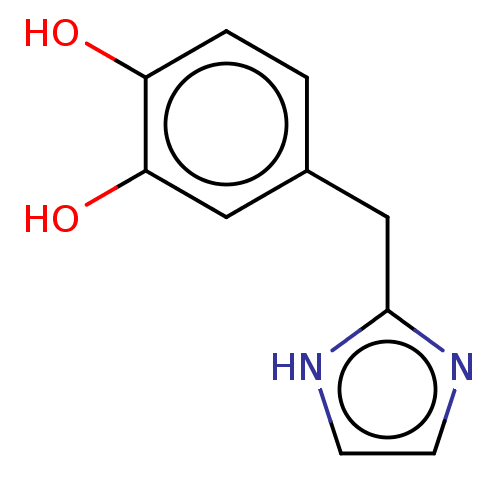
(CHEMBL15228)Show InChI InChI=1S/C11H11N3O/c12-13-11(15)9-3-5-10(6-4-9)14-7-1-2-8-14/h1-8H,12H2,(H,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of AA+phentolamine by the compound in response of SEC |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022536

(CHEMBL15228)Show InChI InChI=1S/C11H11N3O/c12-13-11(15)9-3-5-10(6-4-9)14-7-1-2-8-14/h1-8H,12H2,(H,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of ADP by the compound in response of SEC |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022536

(CHEMBL15228)Show InChI InChI=1S/C11H11N3O/c12-13-11(15)9-3-5-10(6-4-9)14-7-1-2-8-14/h1-8H,12H2,(H,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.61E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of Arachidonic acid by the compound in response of SEC |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022536

(CHEMBL15228)Show InChI InChI=1S/C11H11N3O/c12-13-11(15)9-3-5-10(6-4-9)14-7-1-2-8-14/h1-8H,12H2,(H,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.13E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of U-46,619 + phentolamine by the compound in response of SEC |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50224304

(CHEMBL368261)Show InChI InChI=1S/C17H24N2O2/c1-11(2)19(12(3)4)6-5-13-9-18-15-8-17-16(7-14(13)15)20-10-21-17/h7-9,11-12,18H,5-6,10H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]5-HT binding from 5-hydroxytryptamine receptor site using 1 uM LSD as masking ligand |
J Med Chem 25: 908-13 (1982)
BindingDB Entry DOI: 10.7270/Q2H70J1R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022536

(CHEMBL15228)Show InChI InChI=1S/C11H11N3O/c12-13-11(15)9-3-5-10(6-4-9)14-7-1-2-8-14/h1-8H,12H2,(H,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.55E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Inhibition of U-46,619 by the compound in response of SEC |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022596
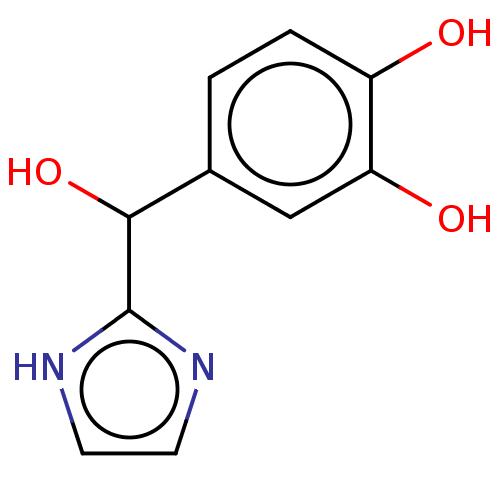
(CHEMBL14837)Show InChI InChI=1S/C34H53N5O6/c1-22(2)16-29(40)30(41)26(17-23-12-8-6-9-13-23)37-32(43)28(19-25-20-35-21-36-25)38-31(42)27(18-24-14-10-7-11-15-24)39-33(44)45-34(3,4)5/h7,10-11,14-15,20-23,26-30,40-41H,6,8-9,12-13,16-19H2,1-5H3,(H,35,36)(H,37,43)(H,38,42)(H,39,44)/t26-,27?,28?,29?,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.29E+12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Stimulatory activity against serotonin secretion (SEC). |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022537
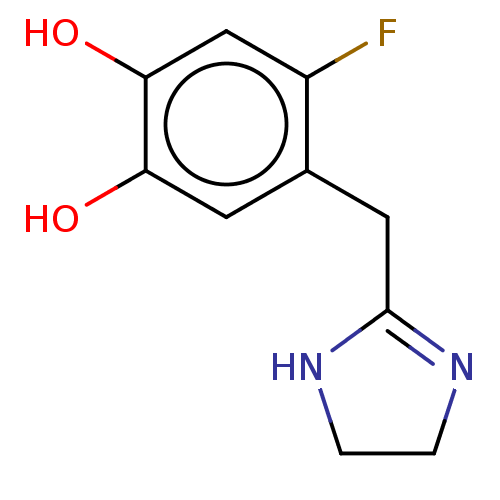
(CHEMBL14508)Show InChI InChI=1S/C11H11N3O/c12-13-11(15)9-4-3-5-10(8-9)14-6-1-2-7-14/h1-8H,12H2,(H,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.63E+13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Stimulatory activity against serotonin secretion (SEC). |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022538
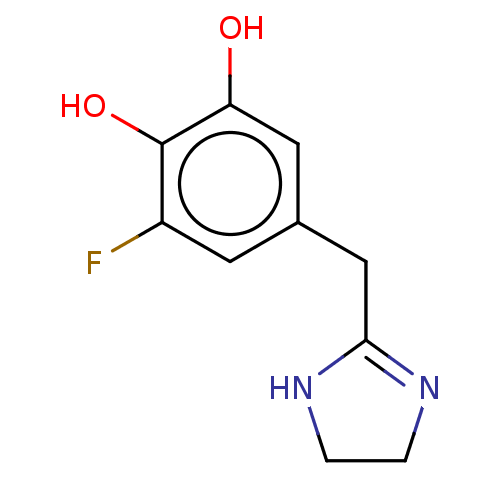
(CHEMBL14651)Show InChI InChI=1S/C11H11N3O/c12-13-11(15)9-5-1-2-6-10(9)14-7-3-4-8-14/h1-8H,12H2,(H,13,15) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.50E+13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Stimulatory activity against serotonin secretion (SEC). |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A/1B/1D/1E/1F/2A/2B/2C/3A/3B/3C/3D/3E/4/5A/6/7
(Homo sapiens (Human)) | BDBM50022539

(CHEMBL14982)Show InChI InChI=1S/C12H13N3O/c13-14-12(16)9-10-4-3-5-11(8-10)15-6-1-2-7-15/h1-8H,9,13H2,(H,14,16) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.46E+13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio State University
Curated by ChEMBL
| Assay Description
Stimulatory activity against serotonin secretion (SEC). |
J Med Chem 33: 1138-44 (1990)
BindingDB Entry DOI: 10.7270/Q2ZC853J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data