 Found 492 hits of ic50 for UniProtKB: P35355
Found 492 hits of ic50 for UniProtKB: P35355 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472616

(CHEMBL65325)Show SMILES CN1CC\C(=C\c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C19H29NO3S/c1-18(2,3)15-11-13(12-16(17(15)21)19(4,5)6)10-14-8-9-20(7)24(14,22)23/h10-12,21H,8-9H2,1-7H3/b14-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472618

(CHEMBL303092)Show SMILES CON1CC\C(=C/c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C19H29NO4S/c1-18(2,3)15-11-13(12-16(17(15)21)19(4,5)6)10-14-8-9-20(24-7)25(14,22)23/h10-12,21H,8-9H2,1-7H3/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472623
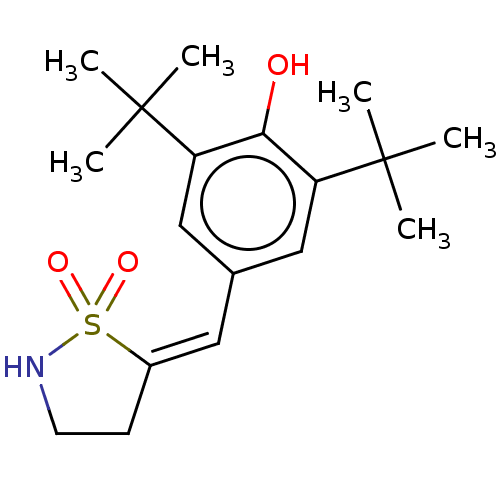
(CHEMBL294100)Show SMILES CC(C)(C)c1cc(\C=C2\CCNS2(=O)=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H27NO3S/c1-17(2,3)14-10-12(9-13-7-8-19-23(13,21)22)11-15(16(14)20)18(4,5)6/h9-11,19-20H,7-8H2,1-6H3/b13-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472632

(CHEMBL65140)Show SMILES CC(=O)N1CC\C(=C/c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C20H29NO4S/c1-13(22)21-9-8-15(26(21,24)25)10-14-11-16(19(2,3)4)18(23)17(12-14)20(5,6)7/h10-12,23H,8-9H2,1-7H3/b15-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472624

(CHEMBL62439)Show SMILES CC(C)(C)c1cc(\C=C2/CCN(c3ccc(Cl)cc3)S2(=O)=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C24H30ClNO3S/c1-23(2,3)20-14-16(15-21(22(20)27)24(4,5)6)13-19-11-12-26(30(19,28)29)18-9-7-17(25)8-10-18/h7-10,13-15,27H,11-12H2,1-6H3/b19-13+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472627
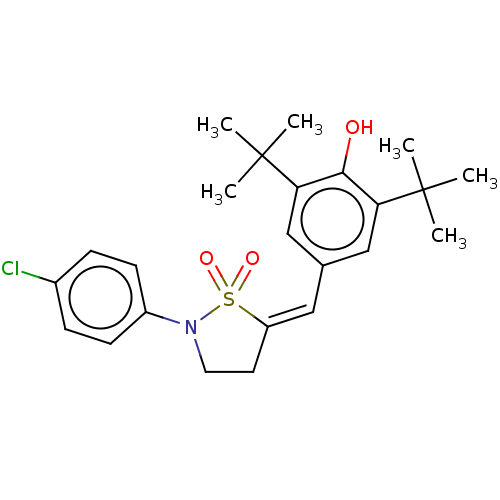
(CHEMBL304489)Show SMILES CC(C)(C)c1cc(\C=C2\CCN(c3ccc(Cl)cc3)S2(=O)=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C24H30ClNO3S/c1-23(2,3)20-14-16(15-21(22(20)27)24(4,5)6)13-19-11-12-26(30(19,28)29)18-9-7-17(25)8-10-18/h7-10,13-15,27H,11-12H2,1-6H3/b19-13- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289087
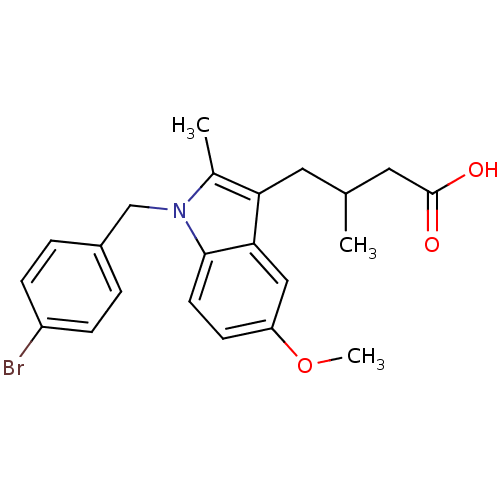
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC(C)CC(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(11-22(25)26)10-19-15(2)24(13-16-4-6-17(23)7-5-16)21-9-8-18(27-3)12-20(19)21/h4-9,12,14H,10-11,13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472613

(CHEMBL305076)Show SMILES CCN1CC\C(=C\c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C20H31NO3S/c1-8-21-10-9-15(25(21,23)24)11-14-12-16(19(2,3)4)18(22)17(13-14)20(5,6)7/h11-13,22H,8-10H2,1-7H3/b15-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472612

(CHEMBL60496)Show SMILES CC(C)(C)c1cc(\C=C2/CCN(C3CC3)S2(=O)=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C21H31NO3S/c1-20(2,3)17-12-14(13-18(19(17)23)21(4,5)6)11-16-9-10-22(15-7-8-15)26(16,24)25/h11-13,15,23H,7-10H2,1-6H3/b16-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472605

(CHEMBL64507)Show SMILES CON1CC\C(=C\c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C19H29NO4S/c1-18(2,3)15-11-13(12-16(17(15)21)19(4,5)6)10-14-8-9-20(24-7)25(14,22)23/h10-12,21H,8-9H2,1-7H3/b14-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289084
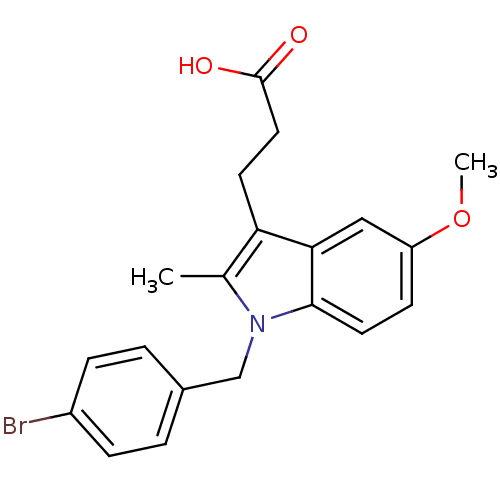
(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCC(O)=O)c2c1 Show InChI InChI=1S/C20H20BrNO3/c1-13-17(8-10-20(23)24)18-11-16(25-2)7-9-19(18)22(13)12-14-3-5-15(21)6-4-14/h3-7,9,11H,8,10,12H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289082
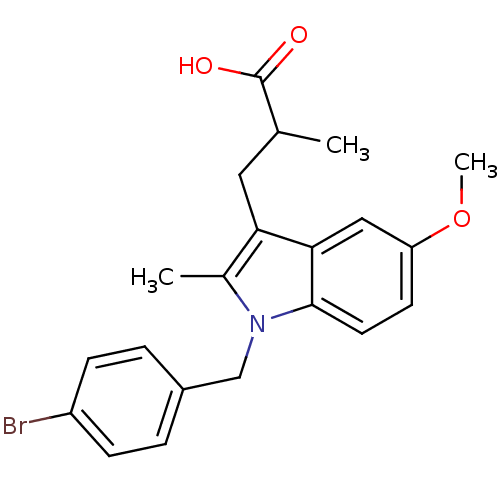
(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC(C)C(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-13(21(24)25)10-18-14(2)23(12-15-4-6-16(22)7-5-15)20-9-8-17(26-3)11-19(18)20/h4-9,11,13H,10,12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289088
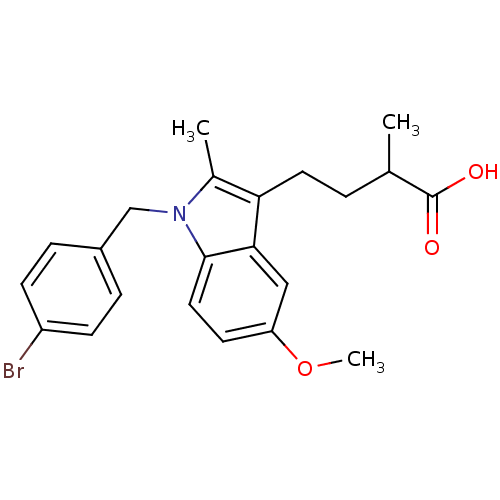
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCC(C)C(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(22(25)26)4-10-19-15(2)24(13-16-5-7-17(23)8-6-16)21-11-9-18(27-3)12-20(19)21/h5-9,11-12,14H,4,10,13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289083

(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(C(C)CC(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-13(10-20(24)25)21-14(2)23(12-15-4-6-16(22)7-5-15)19-9-8-17(26-3)11-18(19)21/h4-9,11,13H,10,12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289081
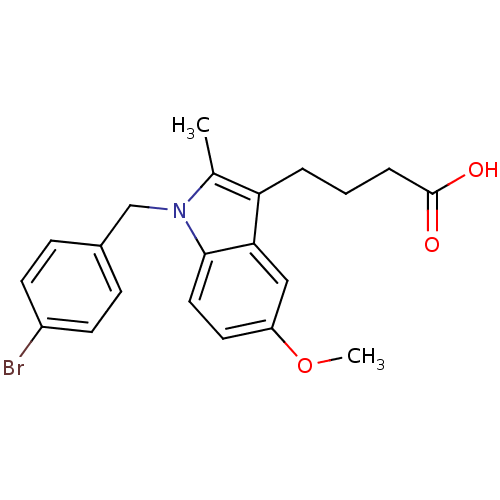
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCCC(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-14-18(4-3-5-21(24)25)19-12-17(26-2)10-11-20(19)23(14)13-15-6-8-16(22)9-7-15/h6-12H,3-5,13H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472615
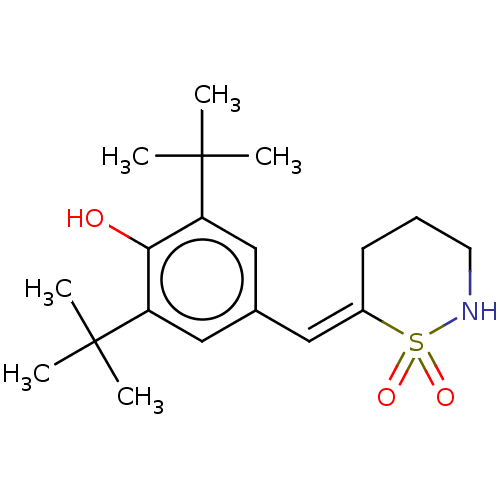
(CHEMBL64813)Show SMILES CC(C)(C)c1cc(\C=C2/CCCNS2(=O)=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C19H29NO3S/c1-18(2,3)15-11-13(12-16(17(15)21)19(4,5)6)10-14-8-7-9-20-24(14,22)23/h10-12,20-21H,7-9H2,1-6H3/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM22970
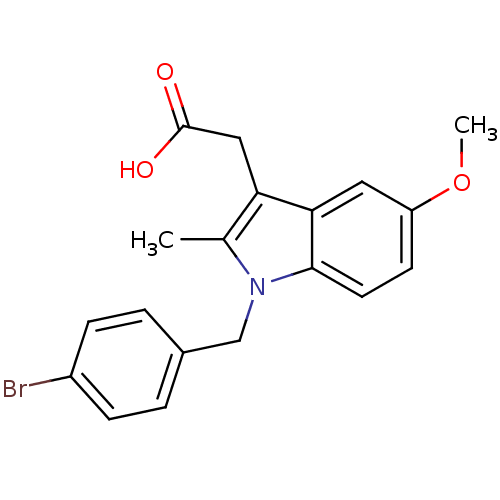
(2-{1-[(4-bromophenyl)methyl]-5-methoxy-2-methyl-1H...)Show InChI InChI=1S/C19H18BrNO3/c1-12-16(10-19(22)23)17-9-15(24-2)7-8-18(17)21(12)11-13-3-5-14(20)6-4-13/h3-9H,10-11H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472610

(CHEMBL60725)Show SMILES CN1CC\C(=C/c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C19H29NO3S/c1-18(2,3)15-11-13(12-16(17(15)21)19(4,5)6)10-14-8-9-20(7)24(14,22)23/h10-12,21H,8-9H2,1-7H3/b14-10+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472622
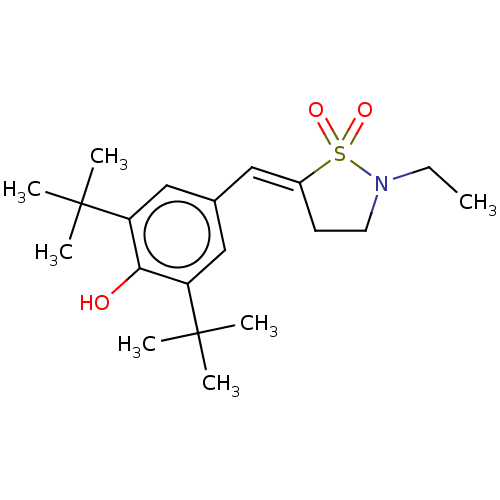
(CHEMBL291543)Show SMILES CCN1CC\C(=C/c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C20H31NO3S/c1-8-21-10-9-15(25(21,23)24)11-14-12-16(19(2,3)4)18(22)17(13-14)20(5,6)7/h11-13,22H,8-10H2,1-7H3/b15-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50331888
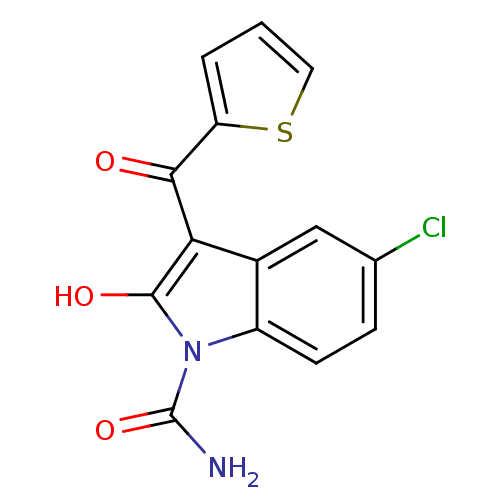
(5-Chloro-2-oxo-3-(thiophene-2-carbonyl)-2,3-dihydr...)Show InChI InChI=1S/C14H9ClN2O3S/c15-7-3-4-9-8(6-7)11(13(19)17(9)14(16)20)12(18)10-2-1-5-21-10/h1-6,19H,(H2,16,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Prostaglandin G/H synthase pathway in rat basophilic leukemia (RBL-1) cells |
J Med Chem 33: 2019-24 (1990)
BindingDB Entry DOI: 10.7270/Q2G73F9X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472631

(CHEMBL60738)Show SMILES CC(C)(C)c1cc(\C=C2/CCN(O)S2(=O)=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H27NO4S/c1-17(2,3)14-10-12(11-15(16(14)20)18(4,5)6)9-13-7-8-19(21)24(13,22)23/h9-11,20-21H,7-8H2,1-6H3/b13-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50076661
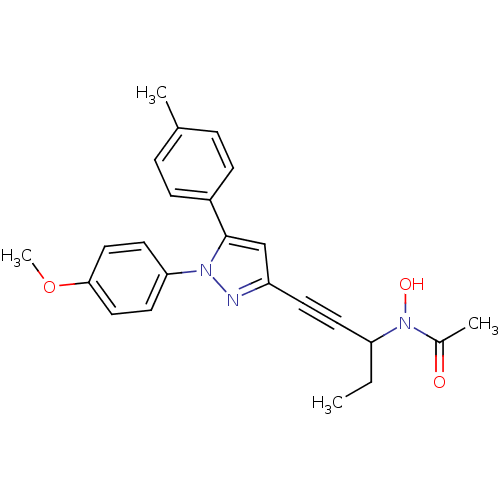
(CHEMBL177727 | N-{1-Ethyl-3-[1-(4-methoxy-phenyl)-...)Show SMILES CCC(C#Cc1cc(-c2ccc(C)cc2)n(n1)-c1ccc(OC)cc1)N(O)C(C)=O Show InChI InChI=1S/C24H25N3O3/c1-5-21(27(29)18(3)28)11-10-20-16-24(19-8-6-17(2)7-9-19)26(25-20)22-12-14-23(30-4)15-13-22/h6-9,12-16,21,29H,5H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) |
Bioorg Med Chem Lett 9: 979-84 (1999)
BindingDB Entry DOI: 10.7270/Q2Z31XV3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50076666
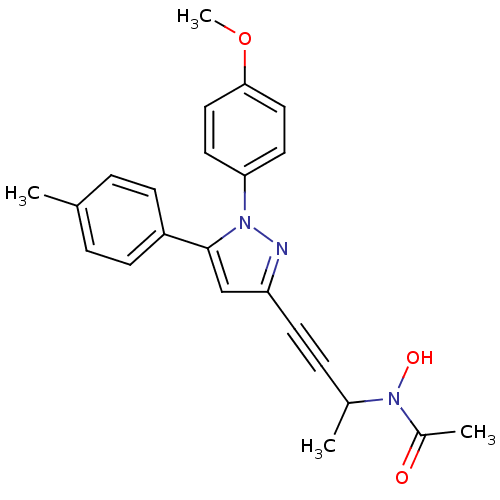
(CHEMBL175153 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(C)cc1)C#CC(C)N(O)C(C)=O Show InChI InChI=1S/C23H23N3O3/c1-16-5-8-19(9-6-16)23-15-20(10-7-17(2)26(28)18(3)27)24-25(23)21-11-13-22(29-4)14-12-21/h5-6,8-9,11-15,17,28H,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) |
Bioorg Med Chem Lett 9: 979-84 (1999)
BindingDB Entry DOI: 10.7270/Q2Z31XV3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50075539

(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-[1,2,4]thia...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nsc(NC#N)n1 Show InChI InChI=1S/C17H22N4OS/c1-16(2,3)11-7-10(8-12(13(11)22)17(4,5)6)14-20-15(19-9-18)23-21-14/h7-8,22H,1-6H3,(H,19,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity against Prostaglandin G/H synthase |
J Med Chem 35: 3691-8 (1992)
BindingDB Entry DOI: 10.7270/Q2M61KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472619
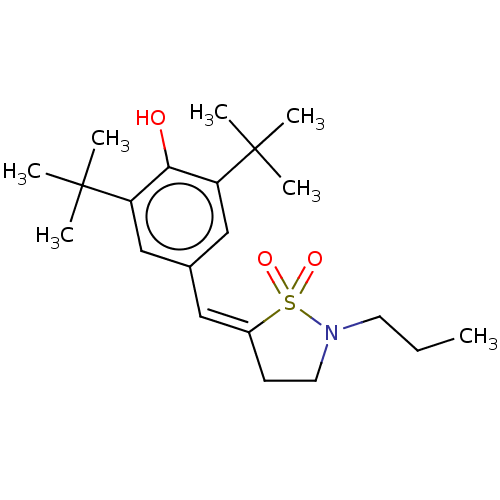
(CHEMBL62990)Show SMILES CCCN1CC\C(=C\c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C21H33NO3S/c1-8-10-22-11-9-16(26(22,24)25)12-15-13-17(20(2,3)4)19(23)18(14-15)21(5,6)7/h12-14,23H,8-11H2,1-7H3/b16-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472625

(CHEMBL62920)Show SMILES CCCN1CC\C(=C/c2cc(c(O)c(c2)C(C)(C)C)C(C)(C)C)S1(=O)=O Show InChI InChI=1S/C21H33NO3S/c1-8-10-22-11-9-16(26(22,24)25)12-15-13-17(20(2,3)4)19(23)18(14-15)21(5,6)7/h12-14,23H,8-11H2,1-7H3/b16-12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289090
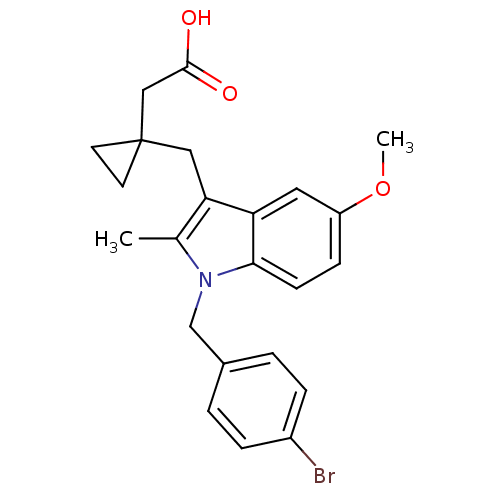
(CHEMBL162776 | {1-[1-(4-Bromo-benzyl)-5-methoxy-2-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC3(CC(O)=O)CC3)c2c1 Show InChI InChI=1S/C23H24BrNO3/c1-15-20(12-23(9-10-23)13-22(26)27)19-11-18(28-2)7-8-21(19)25(15)14-16-3-5-17(24)6-4-16/h3-8,11H,9-10,12-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289089
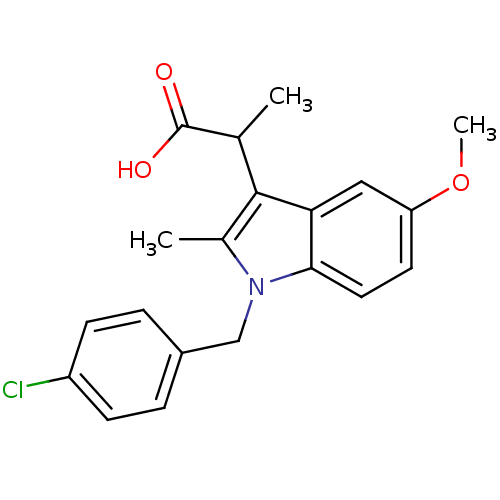
(2-[1-(4-Chloro-benzyl)-5-methoxy-2-methyl-1H-indol...)Show SMILES COc1ccc2n(Cc3ccc(Cl)cc3)c(C)c(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C20H20ClNO3/c1-12(20(23)24)19-13(2)22(11-14-4-6-15(21)7-5-14)18-9-8-16(25-3)10-17(18)19/h4-10,12H,11H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50012860
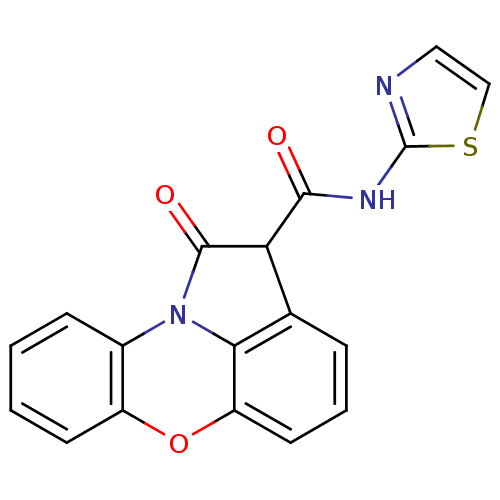
(1-Oxo-1,2-dihydro-6-oxa-10b-aza-aceanthrylene-2-ca...)Show InChI InChI=1S/C18H11N3O3S/c22-16(20-18-19-8-9-25-18)14-10-4-3-7-13-15(10)21(17(14)23)11-5-1-2-6-12(11)24-13/h1-9,14H,(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Prostaglandin G/H synthase pathway in rat basophilic leukemia (RBL-1) cells |
J Med Chem 33: 2019-24 (1990)
BindingDB Entry DOI: 10.7270/Q2G73F9X |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289091

((S)-4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-in...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(C[C@H](C)CC(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(11-22(25)26)10-19-15(2)24(13-16-4-6-17(23)7-5-16)21-9-8-18(27-3)12-20(19)21/h4-9,12,14H,10-11,13H2,1-3H3,(H,25,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472603

(CHEMBL65330)Show SMILES CC(C)(C)c1cc(\C=C2/CCN(C(=O)NO)S2(=O)=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C19H28N2O5S/c1-18(2,3)14-10-12(11-15(16(14)22)19(4,5)6)9-13-7-8-21(17(23)20-24)27(13,25)26/h9-11,22,24H,7-8H2,1-6H3,(H,20,23)/b13-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50223539

(CHEMBL33957)Show InChI InChI=1S/C14H15BrO2/c15-12-6-10-7-14(16)17-13(10)8-11(12)9-4-2-1-3-5-9/h6,8-9H,1-5,7H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin synthesis was tested in rat |
J Med Chem 24: 1465-71 (1981)
BindingDB Entry DOI: 10.7270/Q2M61NFB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50076659

(CHEMBL435907 | N-{3-[5-(4-Chloro-phenyl)-1-(4-meth...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C#CC(C)N(O)C(C)=O Show InChI InChI=1S/C22H20ClN3O3/c1-15(26(28)16(2)27)4-9-19-14-22(17-5-7-18(23)8-6-17)25(24-19)20-10-12-21(29-3)13-11-20/h5-8,10-15,28H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) |
Bioorg Med Chem Lett 9: 979-84 (1999)
BindingDB Entry DOI: 10.7270/Q2Z31XV3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50472609

(CHEMBL64585)Show SMILES CC(C)(C)c1cc(\C=C2/CCNS2(=O)=O)cc(c1O)C(C)(C)C Show InChI InChI=1S/C18H27NO3S/c1-17(2,3)14-10-12(9-13-7-8-19-23(13,21)22)11-15(16(14)20)18(4,5)6/h9-11,19-20H,7-8H2,1-6H3/b13-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50076637
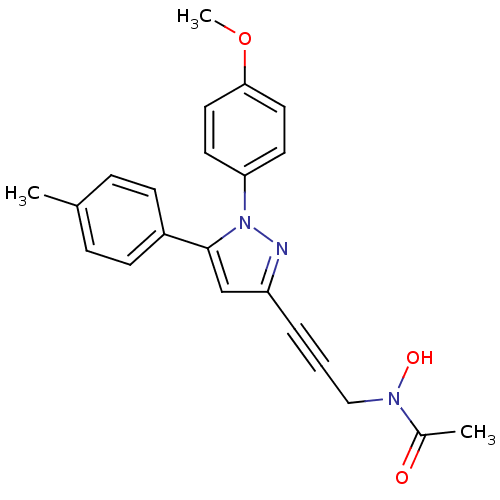
(CHEMBL369252 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(C)cc1)C#CCN(O)C(C)=O Show InChI InChI=1S/C22H21N3O3/c1-16-6-8-18(9-7-16)22-15-19(5-4-14-24(27)17(2)26)23-25(22)20-10-12-21(28-3)13-11-20/h6-13,15,27H,14H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-lipoxygenase (5-LO) in intact rat barophilic leukemia cells (RBL-1) |
Bioorg Med Chem Lett 9: 979-84 (1999)
BindingDB Entry DOI: 10.7270/Q2Z31XV3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50076650
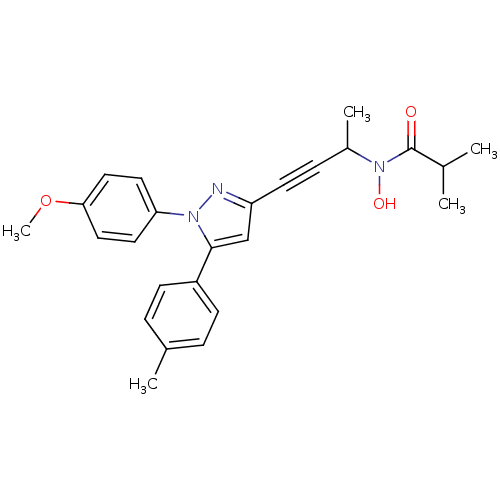
(CHEMBL368682 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(C)cc1)C#CC(C)N(O)C(=O)C(C)C Show InChI InChI=1S/C25H27N3O3/c1-17(2)25(29)28(30)19(4)8-11-21-16-24(20-9-6-18(3)7-10-20)27(26-21)22-12-14-23(31-5)15-13-22/h6-7,9-10,12-17,19,30H,1-5H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against 5-lipoxygenase (5-LO) in intact rat barophilic leukemia cells (RBL-1) |
Bioorg Med Chem Lett 9: 979-84 (1999)
BindingDB Entry DOI: 10.7270/Q2Z31XV3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50279050
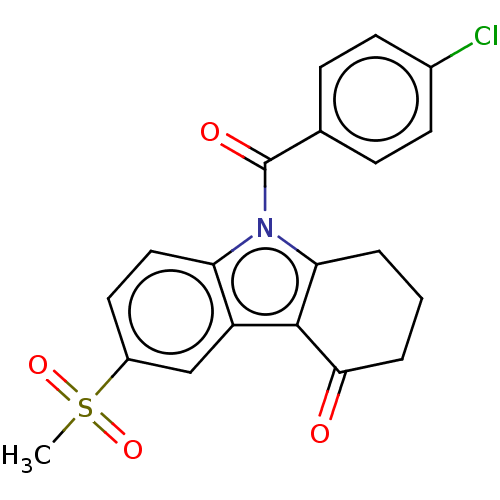
(CHEMBL4169347)Show SMILES CS(=O)(=O)c1ccc2n(C(=O)c3ccc(Cl)cc3)c3CCCC(=O)c3c2c1 Show InChI InChI=1S/C20H16ClNO4S/c1-27(25,26)14-9-10-16-15(11-14)19-17(3-2-4-18(19)23)22(16)20(24)12-5-7-13(21)8-6-12/h5-11H,2-4H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Damanhour University
Curated by ChEMBL
| Assay Description
Binding affinity towards Alpha-1A adrenergic receptor in rat denuded thoracic aorta |
Eur J Med Chem 141: 306-321 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.056
BindingDB Entry DOI: 10.7270/Q2Q242R1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260464

(CHEMBL4071383)Show SMILES CN1C=CN(C=C1)C1NC(=CN1c1ccc(cc1)S(N)(=O)=O)C(F)(F)F |c:2,5,10| Show InChI InChI=1S/C15H16F3N5O2S/c1-21-6-8-22(9-7-21)14-20-13(15(16,17)18)10-23(14)11-2-4-12(5-3-11)26(19,24)25/h2-10,14,20H,1H3,(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50223541
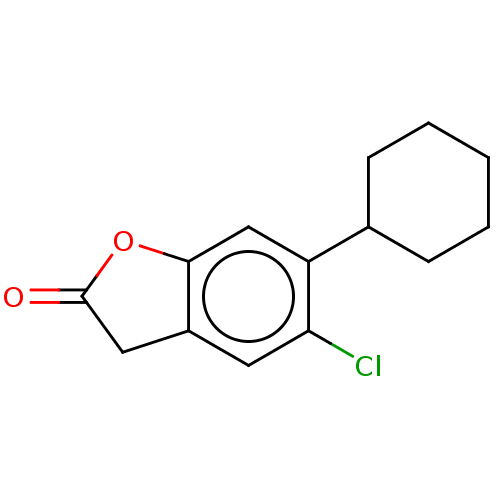
(Clofurac)Show InChI InChI=1S/C14H15ClO2/c15-12-6-10-7-14(16)17-13(10)8-11(12)9-4-2-1-3-5-9/h6,8-9H,1-5,7H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of prostaglandin synthesis was tested in rat |
J Med Chem 24: 1465-71 (1981)
BindingDB Entry DOI: 10.7270/Q2M61NFB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
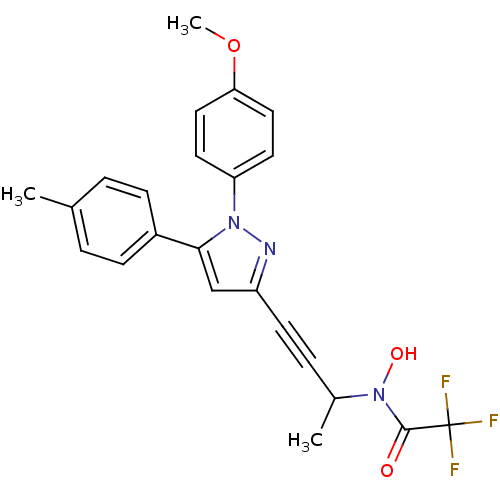
(RAT) | BDBM50076642
(2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc(C)cc1)C#CC(C)N(O)C(=O)C(F)(F)F Show InChI InChI=1S/C23H20F3N3O3/c1-15-4-7-17(8-5-15)21-14-18(9-6-16(2)29(31)22(30)23(24,25)26)27-28(21)19-10-12-20(32-3)13-11-19/h4-5,7-8,10-14,16,31H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) |
Bioorg Med Chem Lett 9: 979-84 (1999)
BindingDB Entry DOI: 10.7270/Q2Z31XV3 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50003117

(3-(3,5-Di-tert-butyl-4-hydroxy-phenyl)-4H-[1,2,4]o...)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1nc(=S)o[nH]1 Show InChI InChI=1S/C16H22N2O2S/c1-15(2,3)10-7-9(13-17-14(21)20-18-13)8-11(12(10)19)16(4,5)6/h7-8,19H,1-6H3,(H,17,18,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Evaluated in vitro for its inhibitory activity against Prostaglandin G/H synthase |
J Med Chem 35: 3691-8 (1992)
BindingDB Entry DOI: 10.7270/Q2M61KWB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289087

(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC(C)CC(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(11-22(25)26)10-19-15(2)24(13-16-4-6-17(23)7-5-16)21-9-8-18(27-3)12-20(19)21/h4-9,12,14H,10-11,13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260465

(CHEMBL4101003)Show SMILES CN1C=CC(C=C1)C1OC(=CN1c1ccc(cc1)S(N)(=O)=O)C(F)(F)F |c:2,5,10| Show InChI InChI=1S/C16H16F3N3O3S/c1-21-8-6-11(7-9-21)15-22(10-14(25-15)16(17,18)19)12-2-4-13(5-3-12)26(20,23)24/h2-11,15H,1H3,(H2,20,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
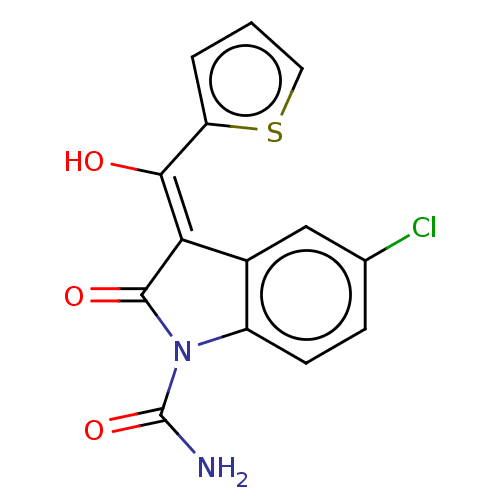
(RAT) | BDBM50472604
(CHEBI:35847 | CP-66,248 | Tenidap)Show SMILES NC(=O)N1C(=O)\C(=C(/O)c2cccs2)c2cc(Cl)ccc12 Show InChI InChI=1S/C14H9ClN2O3S/c15-7-3-4-9-8(6-7)11(13(19)17(9)14(16)20)12(18)10-2-1-5-21-10/h1-6,18H,(H2,16,20)/b12-11- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory effect on production of prostaglandin E2 (PGE2) in rat synovial cells. |
J Med Chem 43: 2040-8 (2000)
Article DOI: 10.1021/jm9906015
BindingDB Entry DOI: 10.7270/Q2H134R8 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
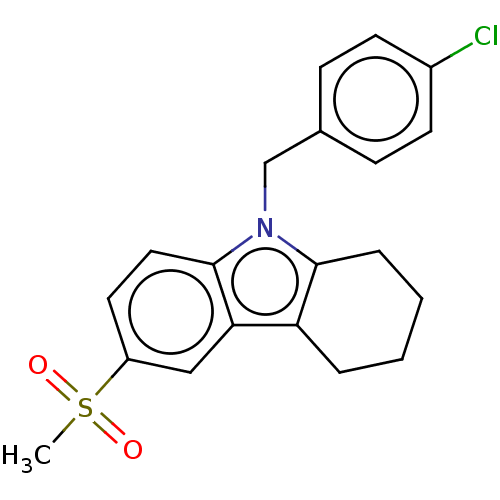
(RAT) | BDBM50279037
(CHEMBL4173526)Show SMILES CS(=O)(=O)c1ccc2n(Cc3ccc(Cl)cc3)c3CCCCc3c2c1 Show InChI InChI=1S/C20H20ClNO2S/c1-25(23,24)16-10-11-20-18(12-16)17-4-2-3-5-19(17)22(20)13-14-6-8-15(21)9-7-14/h6-12H,2-5,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Damanhour University
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against Toxoplasma gondii purine nucleoside phosphorylase of virulent strain RH (value indicates comp... |
Eur J Med Chem 141: 306-321 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.056
BindingDB Entry DOI: 10.7270/Q2Q242R1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50339185
((2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid | ...)Show InChI InChI=1S/C14H14O3/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10/h3-9H,1-2H3,(H,15,16)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Prostaglandin G/H synthase in rat neutrophils |
J Med Chem 29: 1099-113 (1986)
BindingDB Entry DOI: 10.7270/Q2DZ0BHV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1/2
(RAT) | BDBM50076639

(CHEMBL367010 | N-{3-[5-(4-Ethyl-phenyl)-1-(4-metho...)Show SMILES CCc1ccc(cc1)-c1cc(nn1-c1ccc(OC)cc1)C#CC(C)N(O)C(C)=O Show InChI InChI=1S/C24H25N3O3/c1-5-19-7-9-20(10-8-19)24-16-21(11-6-17(2)27(29)18(3)28)25-26(24)22-12-14-23(30-4)15-13-22/h7-10,12-17,29H,5H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) |
Bioorg Med Chem Lett 9: 979-84 (1999)
BindingDB Entry DOI: 10.7270/Q2Z31XV3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data