

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1b receptor (RAT) | BDBM50338810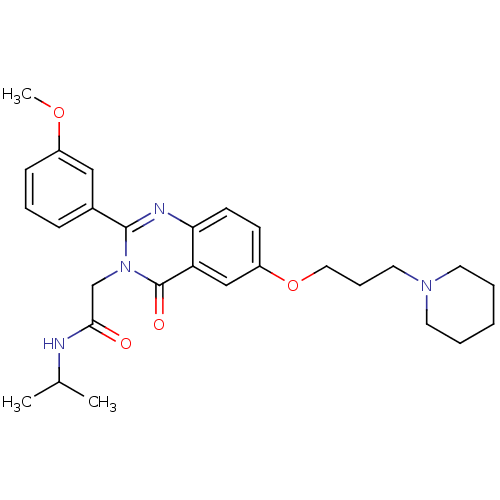 (CHEMBL1684573 | N-isopropyl-2-(2-(3-methoxyphenyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50338811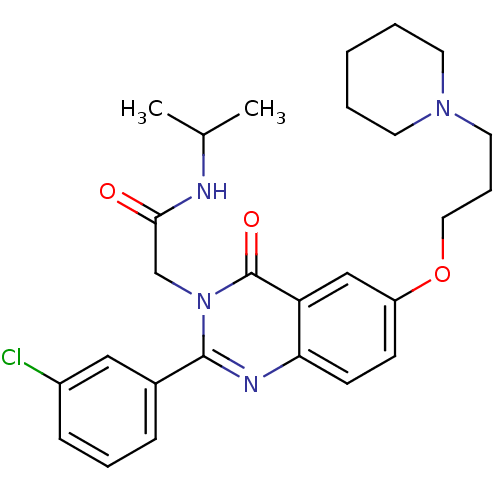 (2-(2-(3-chlorophenyl)-4-oxo-6-(3-(piperidin-1-yl)p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50325847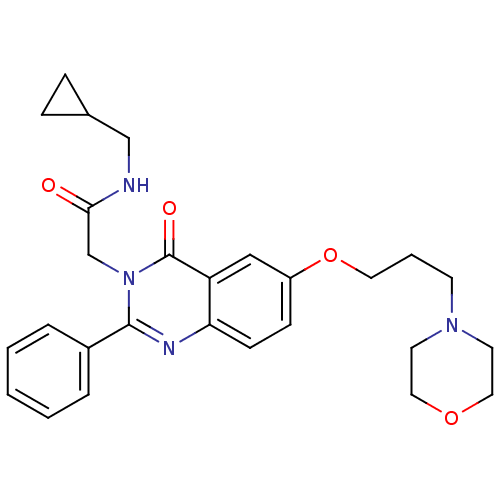 (CHEMBL1223814 | N-(cyclopropylmethyl)-2-(6-(3-morp...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V3 receptor | Bioorg Med Chem Lett 20: 5394-7 (2010) Article DOI: 10.1016/j.bmcl.2010.07.118 BindingDB Entry DOI: 10.7270/Q2222TZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50122716 (5-(Piperidin-4-ylmethoxy)-9-propyl-1,2,3,4-tetrahy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Caen Curated by ChEMBL | Assay Description Ability to displace [3H]AVP from vasopressin V1 receptor | J Med Chem 46: 138-47 (2002) Article DOI: 10.1021/jm020954v BindingDB Entry DOI: 10.7270/Q2J67G8P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||