 Found 19 hits of ic50 for UniProtKB: P15884
Found 19 hits of ic50 for UniProtKB: P15884 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Transcription factor 4
(Homo sapiens) | BDBM50615368

(CHEMBL5285550) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50500849
(CHEMBL3780110)Show SMILES CO[C@@H]1[C@@H](C)OC(NC2=CC(=O)c3c(O)c4C(=O)[C@]5(OC)[C@H](O)Cc6cc(C)c(C(=O)OC)c(O)c6[C@@]5(O)C(=O)c4cc3C2=O)[C@@H](OC)[C@H]1O |r,t:8| Show InChI InChI=1S/C34H35NO15/c1-11-7-13-8-18(37)34(49-6)30(43)21-15(29(42)33(34,45)22(13)25(40)19(11)32(44)48-5)9-14-20(24(21)39)17(36)10-16(23(14)38)35-31-28(47-4)26(41)27(46-3)12(2)50-31/h7,9-10,12,18,26-28,31,35,37,39-41,45H,8H2,1-6H3/t12-,18-,26+,27-,28+,31?,33-,34-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of human GST tagged Tcf4/beta catenin interaction after 2 hrs using fluorescent AP Attophos substrate by ELISA analysis |
Bioorg Med Chem Lett 26: 1664-70 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.064
BindingDB Entry DOI: 10.7270/Q25H7K8M |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM32102
(1,6-dimethylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,...)Show InChI InChI=1S/C7H7N5O2/c1-11-6(13)4-5(10-7(11)14)12(2)9-3-8-4/h3H,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of human GST tagged Tcf4/beta catenin interaction after 2 hrs using fluorescent AP Attophos substrate by ELISA analysis |
Bioorg Med Chem Lett 26: 1664-70 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.064
BindingDB Entry DOI: 10.7270/Q25H7K8M |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50408043
(CHEMBL5278981)Show SMILES [#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6@@H](-[#8])-[#6]-[#6@@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@@H](-[#8])-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-1-[#6]-c2ccccc2-[#6]-[#6@@H]-1-[#6](=O)-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O)-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1 Show InChI InChI=1S/C58H89N19O14S/c59-38(15-6-18-66-56(60)61)48(83)72-39(16-7-19-67-57(62)63)51(86)77-29-36(80)24-45(77)54(89)76-28-35(79)23-43(76)50(85)69-26-46(81)71-41(25-37-14-9-21-92-37)49(84)73-42(31-78)52(87)75-27-33-11-5-4-10-32(33)22-44(75)53(88)74(34-12-2-1-3-13-34)30-47(82)70-40(55(90)91)17-8-20-68-58(64)65/h4-5,9-11,14,21,34-36,38-45,78-80H,1-3,6-8,12-13,15-20,22-31,59H2,(H,69,85)(H,70,82)(H,71,81)(H,72,83)(H,73,84)(H,90,91)(H4,60,61,66)(H4,62,63,67)(H4,64,65,68)/t35-,36-,38-,39-,40-,41-,42-,43+,44+,45+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50556644
(CHEMBL1214274 | NSC-45382)Show SMILES ClC1=C(Nc2ccc(cc2)S(=O)(=O)Nc2ccccn2)C(=O)c2ccccc2C1=O |c:1| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50589224

(CHEMBL5173483) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50158807
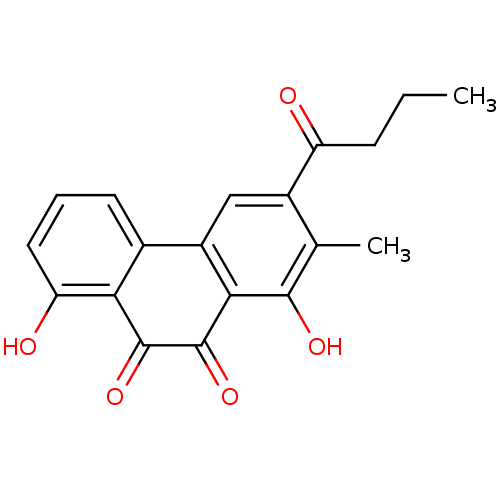
(3-Butyryl-1,8-dihydroxy-2-methyl-phenanthrene-9,10...)Show SMILES CCCC(=O)c1cc2-c3cccc(O)c3C(=O)C(=O)c2c(O)c1C Show InChI InChI=1S/C19H16O5/c1-3-5-13(20)11-8-12-10-6-4-7-14(21)15(10)18(23)19(24)16(12)17(22)9(11)2/h4,6-8,21-22H,3,5H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of human GST tagged Tcf4/beta catenin interaction after 2 hrs using fluorescent AP Attophos substrate by ELISA analysis |
Bioorg Med Chem Lett 26: 1664-70 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.064
BindingDB Entry DOI: 10.7270/Q25H7K8M |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50468575
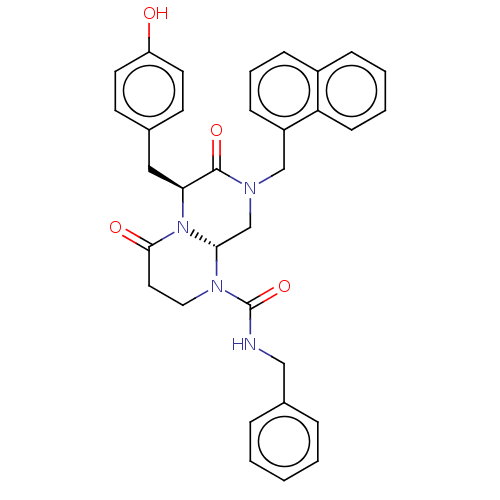
(CHEMBL2312139)Show SMILES [H][C@]12CN(Cc3cccc4ccccc34)C(=O)[C@H](Cc3ccc(O)cc3)N1C(=O)CCN2C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C33H32N4O4/c38-27-15-13-23(14-16-27)19-29-32(40)35(21-26-11-6-10-25-9-4-5-12-28(25)26)22-30-36(18-17-31(39)37(29)30)33(41)34-20-24-7-2-1-3-8-24/h1-16,29-30,38H,17-22H2,(H,34,41)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50500850

(CHEMBL261214)Show SMILES COc1cc(O)c2c3c1c1c(OC)cc(O)c4c1c(c(CC(C)OC(=O)c1ccccc1)c(OC)c4=O)c3c(CC(C)OC(=O)Oc1ccc(O)cc1)c(OC)c2=O Show InChI InChI=1S/C44H38O14/c1-20(56-43(50)22-10-8-7-9-11-22)16-25-31-32-26(17-21(2)57-44(51)58-24-14-12-23(45)13-15-24)42(55-6)40(49)34-28(47)19-30(53-4)36(38(32)34)35-29(52-3)18-27(46)33(37(31)35)39(48)41(25)54-5/h7-15,18-21,45-47H,16-17H2,1-6H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of human GST tagged Tcf4/beta catenin interaction after 2 hrs using fluorescent AP Attophos substrate by ELISA analysis |
Bioorg Med Chem Lett 26: 1664-70 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.064
BindingDB Entry DOI: 10.7270/Q25H7K8M |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50590853
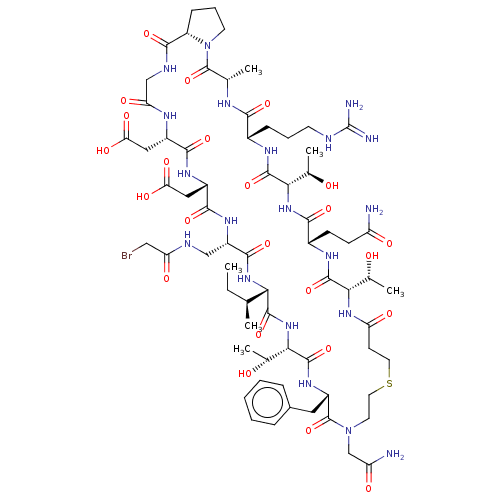
(CHEMBL5172034)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)CCSCCN(CC(N)=O)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CNC(=O)CBr)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC2=O)[C@@H](C)CC)[C@@H](C)O)[C@@H](C)O)[C@@H](C)O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01077
BindingDB Entry DOI: 10.7270/Q24Q7ZZW |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50500848

(CHEMBL2397075)Show SMILES COc1cc(O)c2c3c1c1c(OC)cc(O)c4c1c(c(CC(C)OC(=O)c1c(C)cc(O)cc1O)c(OC)c4=O)c3c(CC(C)OC(=O)c1c(C)cc(O)cc1O)c(OC)c2=O Show InChI InChI=1S/C46H42O16/c1-17-9-21(47)13-25(49)31(17)45(55)61-19(3)11-23-33-34-24(12-20(4)62-46(56)32-18(2)10-22(48)14-26(32)50)44(60-8)42(54)36-28(52)16-30(58-6)38(40(34)36)37-29(57-5)15-27(51)35(39(33)37)41(53)43(23)59-7/h9-10,13-16,19-20,47-52H,11-12H2,1-8H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King Saud University
Curated by ChEMBL
| Assay Description
Inhibition of human GST tagged Tcf4/beta catenin interaction after 2 hrs using fluorescent AP Attophos substrate by ELISA analysis |
Bioorg Med Chem Lett 26: 1664-70 (2016)
Article DOI: 10.1016/j.bmcl.2016.02.064
BindingDB Entry DOI: 10.7270/Q25H7K8M |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50408046

(CHEMBL5284392)Show SMILES [#7]-[#6](-[#7])-[#7]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-1-[#6]-[#6@H](-[#8])-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-c1cccs1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7](-[#6]-[#6](=O)-[#7]-1-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-2-[#6]-[#6@H]-1-[#6](-[#8])=O)-[#6]-c1ccccc1 Show InChI InChI=1S/C52H79N15O12S/c53-34(14-6-18-58-51(54)55)44(72)62-35(15-7-19-59-52(56)57)48(76)65-20-8-17-39(65)49(77)66-27-32(69)23-40(66)46(74)60-25-42(70)61-36(24-33-13-9-21-80-33)45(73)63-37(29-68)47(75)64(26-30-10-2-1-3-11-30)28-43(71)67-38-16-5-4-12-31(38)22-41(67)50(78)79/h1-3,9-11,13,21,31-32,34-41,52,59,68-69H,4-8,12,14-20,22-29,53,56-57H2,(H,60,74)(H,61,70)(H,62,72)(H,63,73)(H,78,79)(H4,54,55,58)/t31?,32-,34-,35+,36+,37+,38?,39+,40+,41+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50468575

(CHEMBL2312139)Show SMILES [H][C@]12CN(Cc3cccc4ccccc34)C(=O)[C@H](Cc3ccc(O)cc3)N1C(=O)CCN2C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C33H32N4O4/c38-27-15-13-23(14-16-27)19-29-32(40)35(21-26-11-6-10-25-9-4-5-12-28(25)26)22-30-36(18-17-31(39)37(29)30)33(41)34-20-24-7-2-1-3-8-24/h1-16,29-30,38H,17-22H2,(H,34,41)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Affinity for CCK2 receptor assessed by inhibition of pentagastrin-stimulated acid secretion in perfused rat stomach |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50590852

(CHEMBL5202327)Show SMILES [H][C@@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)[C@@H](NC(=O)[C@@H]1CSCc3cccc(CSC[C@H](NC(=O)[C@H](CC(O)=O)NC2=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc2ccccc2)C(=O)NCCC(=O)NCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(N)=O)C(=O)N1)c3)C(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01077
BindingDB Entry DOI: 10.7270/Q24Q7ZZW |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50589220

(CHEMBL3890384)Show SMILES [H][C@](C)(Cc1c(OC)c(=O)c2c(O)cc(OC)c3c4c(OC)cc(O)c5c4c(c(C[C@]([H])(C)OC(=O)c4ccccc4)c(OC)c5=O)c1c23)OC(=O)Oc1ccc(O)cc1 |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50094484
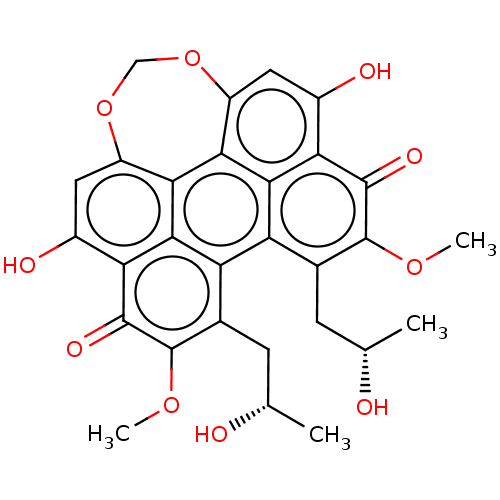
(CHEBI:3556 | CHEMBL2323033 | US9284299, CGP049090 ...)Show SMILES COc1c(C[C@H](C)O)c2c3c(C[C@H](C)O)c(OC)c(=O)c4c(O)cc5OCOc6cc(O)c(c2c6c5c34)c1=O |r| Show InChI InChI=1S/C27H29NO3/c29-26(27(30,23-12-6-2-7-13-23)24-14-8-3-9-15-24)31-25-17-20-28(21-18-25)19-16-22-10-4-1-5-11-22/h1-15,25,30H,16-21H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50589221

(CHEMBL5192638)Show SMILES COc1cc(O)c2c3c1c1c(OC)cc(O)c4c1c(c(C[C@H](C)OC(=O)c1c(C)cc(O)cc1O)c(OC)c4=O)c3c(C[C@H](C)OC(=O)c1c(C)cc(O)cc1O)c(OC)c2=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00228
BindingDB Entry DOI: 10.7270/Q2GQ72Q3 |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50589363
(CHEMBL5191201)Show SMILES [H][C@@]12CCCN1C(=O)[C@]1([H])CCCN1C(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCNC(=O)CCNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC2=O)C(C)C)C(C)C)[C@@H](C)O)C(C)C |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01077
BindingDB Entry DOI: 10.7270/Q24Q7ZZW |
More data for this
Ligand-Target Pair | |
Transcription factor 4
(Homo sapiens) | BDBM50589362

(CHEMBL5181203)Show SMILES CCc1ccc(OCc2nnc(SC3CCCC3=O)n2C(C)c2ccccc2)cc1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116920
BindingDB Entry DOI: 10.7270/Q2T157M8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data