 Found 34 hits of ic50 for UniProtKB: P13584
Found 34 hits of ic50 for UniProtKB: P13584 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 4B1
(Homo sapiens) | BDBM50591915

(CHEMBL2130955) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114332
BindingDB Entry DOI: 10.7270/Q20P1410 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50591915

(CHEMBL2130955) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114332
BindingDB Entry DOI: 10.7270/Q20P1410 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50591915

(CHEMBL2130955) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114332
BindingDB Entry DOI: 10.7270/Q20P1410 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50591915

(CHEMBL2130955) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114332
BindingDB Entry DOI: 10.7270/Q20P1410 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50388532
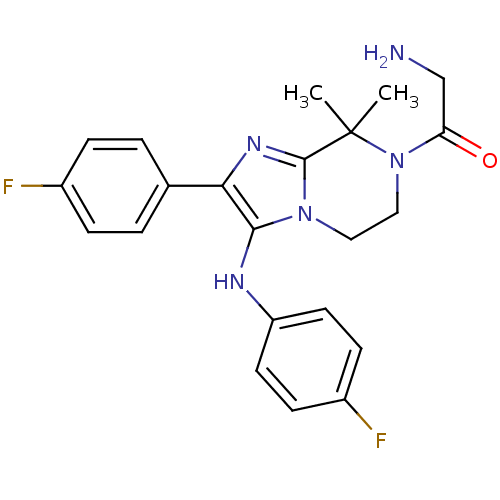
(CHEMBL2058833)Show SMILES CC1(C)N(CCn2c(Nc3ccc(F)cc3)c(nc12)-c1ccc(F)cc1)C(=O)CN Show InChI InChI=1S/C22H23F2N5O/c1-22(2)21-27-19(14-3-5-15(23)6-4-14)20(26-17-9-7-16(24)8-10-17)28(21)11-12-29(22)18(30)13-25/h3-10,26H,11-13,25H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112955
BindingDB Entry DOI: 10.7270/Q2S75M9J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50508662

(CHEMBL4435025)Show SMILES Cc1cn(C2CCCCC2)c2ccc(cc12)C(=O)NS(=O)(=O)C1CC1 Show InChI InChI=1S/C19H24N2O3S/c1-13-12-21(15-5-3-2-4-6-15)18-10-7-14(11-17(13)18)19(22)20-25(23,24)16-8-9-16/h7,10-12,15-16H,2-6,8-9H2,1H3,(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP450 |
Bioorg Med Chem Lett 29: 659-663 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.013
BindingDB Entry DOI: 10.7270/Q2K64NC8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50244440
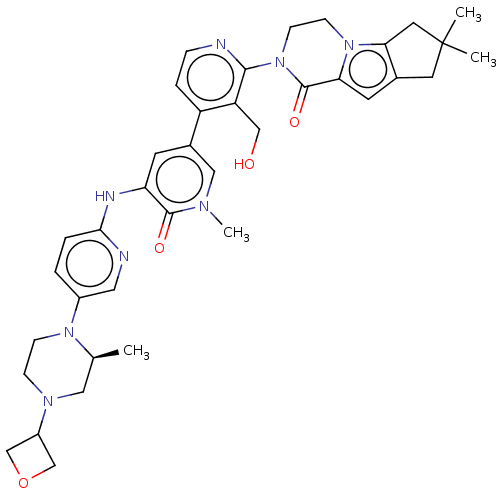
(CHEMBL4065122)Show SMILES C[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human liver microsome CYP450 |
ACS Med Chem Lett 11: 1588-1597 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00249
BindingDB Entry DOI: 10.7270/Q23J3HJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50505550

(CHEMBL4439190 | US11260049, Ex. 2)Show SMILES OC[C@]1(O)CN(C[C@@H]1S(=O)(=O)c1ccc(Cl)cc1)S(=O)(=O)c1ccc(cc1Cl)C#N |r| Show InChI InChI=1S/C18H16Cl2N2O6S2/c19-13-2-4-14(5-3-13)29(25,26)17-9-22(10-18(17,24)11-23)30(27,28)16-6-1-12(8-21)7-15(16)20/h1-7,17,23-24H,9-11H2/t17-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
J Med Chem 61: 11209-11220 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01344
BindingDB Entry DOI: 10.7270/Q2KW5KD5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50543383
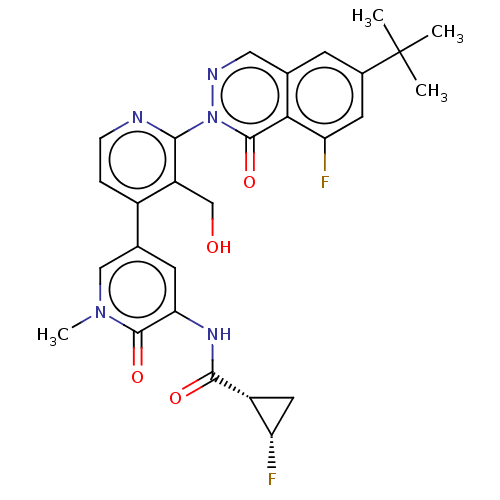
(CHEMBL4634904)Show SMILES Cn1cc(cc(NC(=O)[C@@H]2C[C@@H]2F)c1=O)-c1ccnc(c1CO)-n1ncc2cc(cc(F)c2c1=O)C(C)(C)C |r| Show InChI InChI=1S/C28H27F2N5O4/c1-28(2,3)16-7-14-11-32-35(27(39)23(14)21(30)9-16)24-19(13-36)17(5-6-31-24)15-8-22(26(38)34(4)12-15)33-25(37)18-10-20(18)29/h5-9,11-12,18,20,36H,10,13H2,1-4H3,(H,33,37)/t18-,20+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human liver microsome CYP450 |
ACS Med Chem Lett 11: 1588-1597 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00249
BindingDB Entry DOI: 10.7270/Q23J3HJK |
More data for this
Ligand-Target Pair | |
Cytochrome P450
(Homo sapiens (Human)) | BDBM50508661

(CHEMBL4524830)Show SMILES Cn1cc(C2CCCCC2)c2cc(F)c(cc12)C(=O)NS(=O)(=O)C1CC1 Show InChI InChI=1S/C19H23FN2O3S/c1-22-11-16(12-5-3-2-4-6-12)14-9-17(20)15(10-18(14)22)19(23)21-26(24,25)13-7-8-13/h9-13H,2-8H2,1H3,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CYP450 |
Bioorg Med Chem Lett 29: 659-663 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.013
BindingDB Entry DOI: 10.7270/Q2K64NC8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50550026
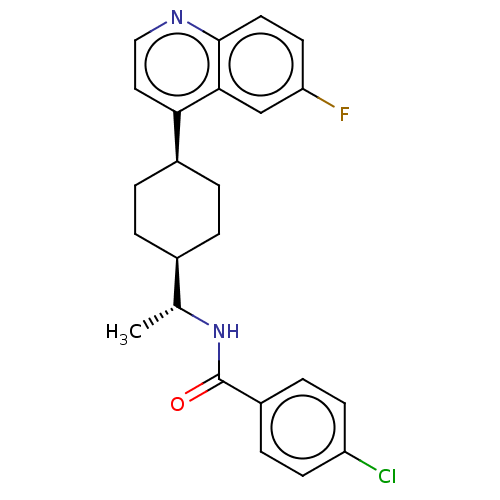
(CHEMBL4786690)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)NC(=O)c1ccc(Cl)cc1 |r,wU:4.7,18.21,wD:1.0,(55.56,-25.24,;55.58,-26.78,;54.24,-26.01,;52.9,-26.78,;52.91,-28.32,;54.25,-29.09,;55.57,-28.32,;51.57,-29.08,;50.24,-28.32,;48.91,-29.1,;48.92,-30.63,;50.25,-31.38,;50.25,-32.93,;51.58,-33.7,;52.92,-32.93,;54.25,-33.69,;52.91,-31.38,;51.58,-30.61,;56.91,-26.01,;56.91,-24.47,;58.24,-26.78,;58.24,-28.32,;56.9,-29.08,;59.58,-29.09,;59.57,-30.63,;60.9,-31.4,;62.24,-30.63,;63.57,-31.4,;62.23,-29.09,;60.9,-28.32,)| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CYP450 |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00668
BindingDB Entry DOI: 10.7270/Q2TM7FR5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50017716
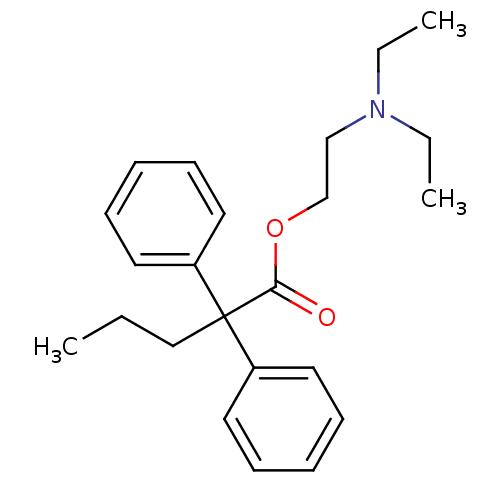
(2,2-Diphenyl-pentanoic acid 2-diethylamino-ethyl e...)Show InChI InChI=1S/C23H31NO2/c1-4-17-23(20-13-9-7-10-14-20,21-15-11-8-12-16-21)22(25)26-19-18-24(5-2)6-3/h7-16H,4-6,17-19H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
J Med Chem 63: 10135-10157 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02038
BindingDB Entry DOI: 10.7270/Q2S46WJB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50512919
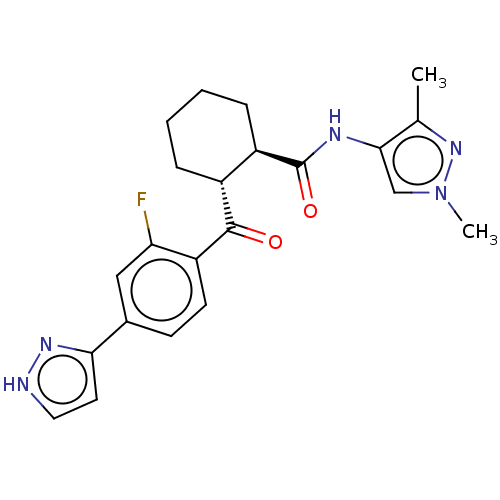
(CHEMBL4554158)Show SMILES Cc1nn(C)cc1NC(=O)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1F)-c1cc[nH]n1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-13-20(12-28(2)27-13)25-22(30)16-6-4-3-5-15(16)21(29)17-8-7-14(11-18(17)23)19-9-10-24-26-19/h7-12,15-16H,3-6H2,1-2H3,(H,24,26)(H,25,30)/t15-,16-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 assessed as maximum reduction in metabolite formation using coumarin based substrate by fluorescence assay |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50512922

(CHEMBL4533700)Show SMILES O=C(Nc1cnn2cccnc12)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C23H22N6O2/c30-21(16-8-6-15(7-9-16)19-10-12-25-28-19)17-4-1-2-5-18(17)23(31)27-20-14-26-29-13-3-11-24-22(20)29/h3,6-14,17-18H,1-2,4-5H2,(H,25,28)(H,27,31)/t17-,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 assessed as maximum reduction in metabolite formation using coumarin based substrate by fluorescence assay |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50512926
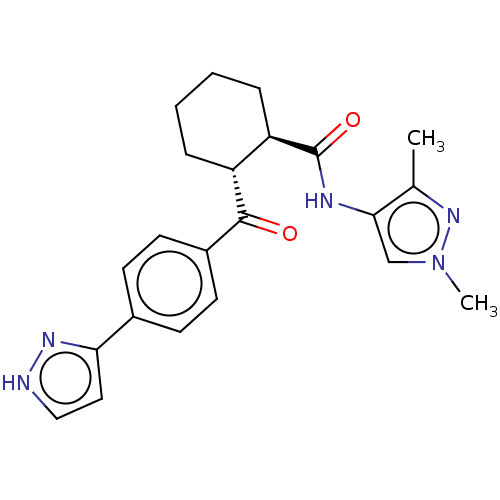
(CHEMBL4554389)Show SMILES Cc1nn(C)cc1NC(=O)[C@@H]1CCCC[C@H]1C(=O)c1ccc(cc1)-c1cc[nH]n1 |r| Show InChI InChI=1S/C22H25N5O2/c1-14-20(13-27(2)26-14)24-22(29)18-6-4-3-5-17(18)21(28)16-9-7-15(8-10-16)19-11-12-23-25-19/h7-13,17-18H,3-6H2,1-2H3,(H,23,25)(H,24,29)/t17-,18-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 assessed as maximum reduction in metabolite formation using coumarin based substrate by fluorescence assay |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50541296
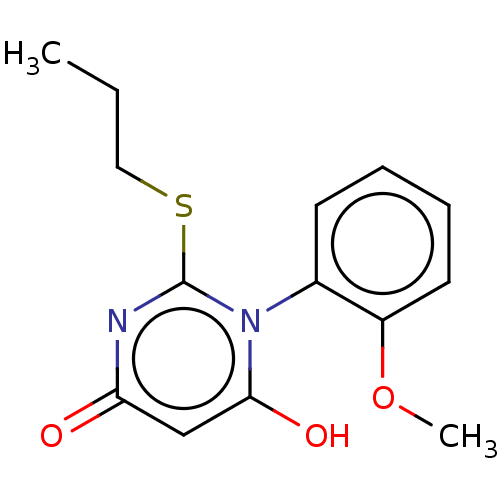
(CHEMBL4636169)Show SMILES CCCSc1nc(=O)cc(O)n1-c1ccccc1OC |(38.91,-20.71,;40.24,-21.48,;41.58,-20.71,;42.91,-21.47,;44.24,-20.7,;44.25,-19.16,;45.58,-18.39,;45.58,-16.85,;46.91,-19.16,;46.91,-20.69,;48.25,-21.46,;45.58,-21.46,;45.58,-23,;44.25,-23.77,;44.25,-25.31,;45.58,-26.08,;46.92,-25.31,;46.91,-23.76,;48.24,-22.99,;49.58,-23.75,)| Show InChI InChI=1S/C14H16N2O3S/c1-3-8-20-14-15-12(17)9-13(18)16(14)10-6-4-5-7-11(10)19-2/h4-7,9,18H,3,8H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126955
BindingDB Entry DOI: 10.7270/Q27W6GRP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50541318
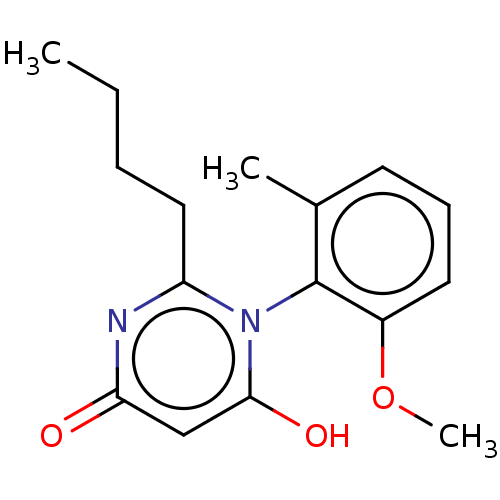
(CHEMBL4632688)Show SMILES CCCCc1nc(=O)cc(O)n1-c1c(C)cccc1OC |(56.29,-35.76,;57.62,-36.53,;58.95,-35.76,;60.29,-36.53,;61.62,-35.75,;61.62,-34.21,;62.96,-33.44,;62.96,-31.9,;64.29,-34.21,;64.29,-35.74,;65.63,-36.51,;62.96,-36.51,;62.96,-38.05,;64.29,-38.81,;65.62,-38.04,;64.3,-40.36,;62.96,-41.13,;61.63,-40.36,;61.63,-38.82,;60.29,-38.05,;58.96,-38.82,)| Show InChI InChI=1S/C16H20N2O3/c1-4-5-9-13-17-14(19)10-15(20)18(13)16-11(2)7-6-8-12(16)21-3/h6-8,10,20H,4-5,9H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126955
BindingDB Entry DOI: 10.7270/Q27W6GRP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50589165
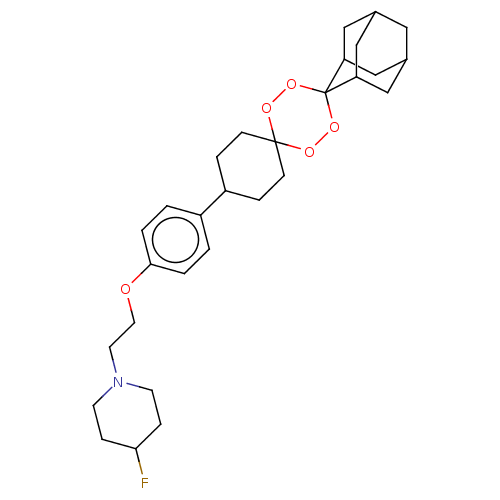
(CHEMBL4288940)Show SMILES FC1CCN(CCOc2ccc(cc2)C2CCC3(CC2)OOC2(OO3)C3CC4CC(C3)CC2C4)CC1 |TLB:30:29:33:26.25.22,30:25:28.29.31:33,THB:22:25:28:31.32.33,22:32:28:26.30.25| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112955
BindingDB Entry DOI: 10.7270/Q2S75M9J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50613392
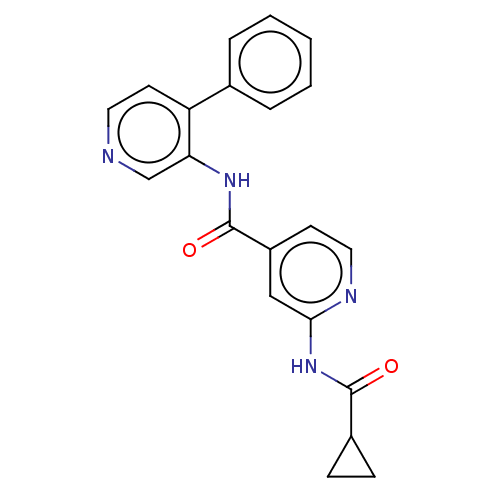
(CHEMBL5270581) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50365230

(CHEMBL1956285 | US11903936, Compound DSM265 | US92...)Show SMILES Cc1cc(Nc2ccc(cc2)S(F)(F)(F)(F)F)n2nc(nc2n1)C(C)(F)F Show InChI InChI=1S/C14H12F7N5S/c1-8-7-11(26-13(22-8)24-12(25-26)14(2,15)16)23-9-3-5-10(6-4-9)27(17,18,19,20)21/h3-7,23H,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112955
BindingDB Entry DOI: 10.7270/Q2S75M9J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM368625
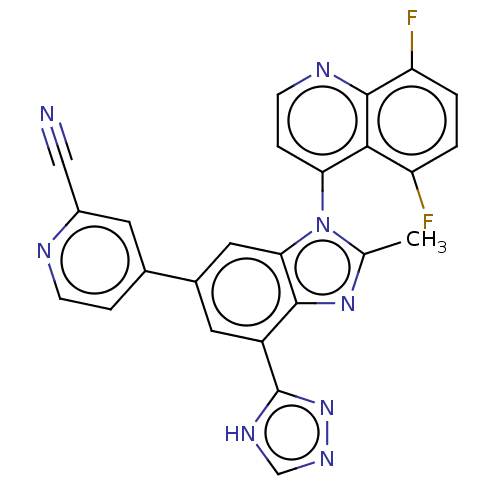
(US10227350, Compound 113 | US10227350, Compound 11...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(c1)C#N)-c1nnc[nH]1 |(-5.8,-.24,;-4.26,-.24,;-3.78,-1.7,;-2.24,-1.7,;-1.21,-2.85,;.3,-2.53,;.77,-1.06,;-.26,.08,;-1.77,-.24,;-3.01,.67,;-3.01,2.21,;-1.68,2.98,;-1.68,4.52,;-3.01,5.29,;-4.35,4.52,;-5.68,5.29,;-5.68,6.83,;-7.01,4.52,;-7.01,2.98,;-5.68,2.21,;-5.68,.67,;-4.35,2.98,;2.26,-.66,;2.66,.82,;4.14,1.22,;5.23,.13,;4.83,-1.35,;3.35,-1.75,;5.92,-2.44,;7.01,-3.53,;-1.61,-4.33,;-.7,-5.58,;-1.61,-6.83,;-3.07,-6.35,;-3.07,-4.81,)| Show InChI InChI=1S/C25H14F2N8/c1-13-33-23-17(25-31-12-32-34-25)9-15(14-4-6-29-16(8-14)11-28)10-21(23)35(13)20-5-7-30-24-19(27)3-2-18(26)22(20)24/h2-10,12H,1H3,(H,31,32,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP450 |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM368657

(US10227350, Compound 105 | US10227350, Compound 10...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(N)c1F)-c1nnc[nH]1 |(-5.25,-.24,;-3.71,-.24,;-3.24,-1.7,;-1.7,-1.7,;-.67,-2.85,;.84,-2.53,;1.32,-1.06,;.28,.08,;-1.22,-.24,;-2.47,.67,;-2.47,2.21,;-1.13,2.98,;-1.13,4.52,;-2.47,5.29,;-3.8,4.52,;-5.13,5.29,;-5.13,6.83,;-6.47,4.52,;-6.47,2.98,;-5.13,2.21,;-5.13,.67,;-3.8,2.98,;2.8,-.66,;3.2,.82,;4.69,1.22,;5.78,.13,;5.38,-1.35,;6.47,-2.44,;3.89,-1.75,;3.49,-3.24,;-1.07,-4.33,;-.16,-5.58,;-1.07,-6.83,;-2.53,-6.35,;-2.53,-4.81,)| Show InChI InChI=1S/C24H15F3N8/c1-11-33-21-14(24-31-10-32-34-24)8-12(13-4-6-30-23(28)20(13)27)9-18(21)35(11)17-5-7-29-22-16(26)3-2-15(25)19(17)22/h2-10H,1H3,(H2,28,30)(H,31,32,34) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP450 |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50511451
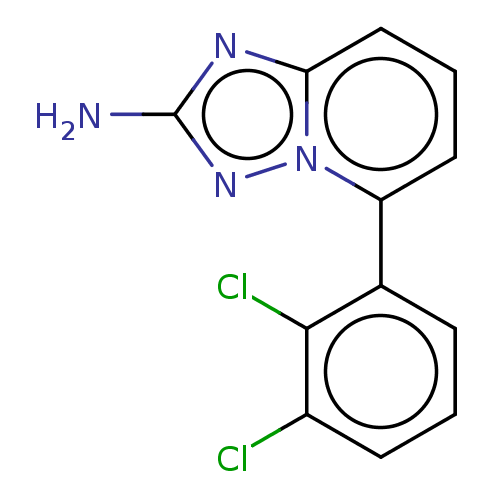
(CHEMBL4570428)Show InChI InChI=1S/C12H8Cl2N4/c13-8-4-1-3-7(11(8)14)9-5-2-6-10-16-12(15)17-18(9)10/h1-6H,(H2,15,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP isoform (unknown origin) |
ACS Med Chem Lett 11: 358-364 (2020)
Article DOI: 10.1021/acsmedchemlett.9b00420
BindingDB Entry DOI: 10.7270/Q2PV6PPZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50512931

(CHEMBL4528928)Show SMILES Cc1cc(n[nH]1)-c1ccc(cc1)C(=O)[C@@H]1CCCC[C@H]1C(=O)Nc1cn(C)nc1C |r| Show InChI InChI=1S/C23H27N5O2/c1-14-12-20(26-25-14)16-8-10-17(11-9-16)22(29)18-6-4-5-7-19(18)23(30)24-21-13-28(3)27-15(21)2/h8-13,18-19H,4-7H2,1-3H3,(H,24,30)(H,25,26)/t18-,19-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 assessed as maximum reduction in metabolite formation using coumarin based substrate by fluorescence assay |
J Med Chem 62: 4325-4349 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02012
BindingDB Entry DOI: 10.7270/Q2S75KP2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM330295
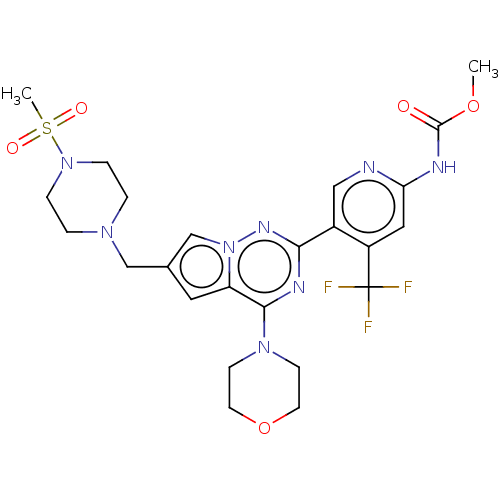
(Methyl (5-(6-((4-(methylsulfonyl)piperazin-1-yl)me...)Show SMILES COC(=O)Nc1cc(c(cn1)-c1nc(N2CCOCC2)c2cc(CN3CCN(CC3)S(C)(=O)=O)cn2n1)C(F)(F)F Show InChI InChI=1S/C24H29F3N8O5S/c1-39-23(36)29-20-12-18(24(25,26)27)17(13-28-20)21-30-22(33-7-9-40-10-8-33)19-11-16(15-35(19)31-21)14-32-3-5-34(6-4-32)41(2,37)38/h11-13,15H,3-10,14H2,1-2H3,(H,28,29,36) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP450 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112913
BindingDB Entry DOI: 10.7270/Q2TF0228 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM264212
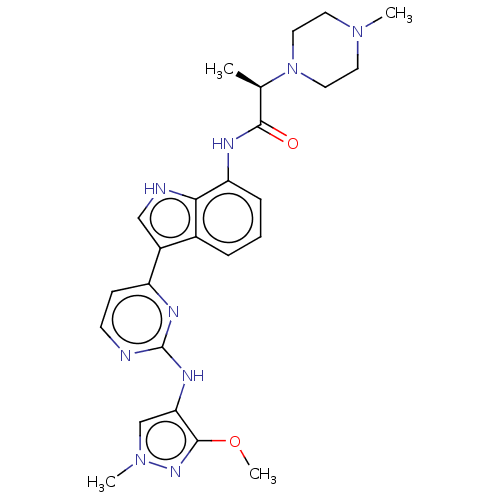
((2R)-N-(3-{2-[(3- methoxy-1-methyl- 1H-pyrazol-4-y...)Show SMILES COc1nn(C)cc1Nc1nccc(n1)-c1c[nH]c2c(NC(=O)[C@@H](C)N3CCN(C)CC3)cccc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human Cytochrome P450 |
J Med Chem 63: 4517-4527 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01392
BindingDB Entry DOI: 10.7270/Q21V5JDF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM413450

(7-methyl-2-((7-methyl-[1,2,4]triazolo[1,5-a]pyridi...)Show SMILES Cc1cc2ncnn2cc1Nc1ncc2n(C)c(=O)n(C3CCOCC3)c2n1 Show InChI InChI=1S/C18H20N8O2/c1-11-7-15-20-10-21-25(15)9-13(11)22-17-19-8-14-16(23-17)26(18(27)24(14)2)12-3-5-28-6-4-12/h7-10,12H,3-6H2,1-2H3,(H,19,22,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
J Med Chem 63: 3461-3471 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01684
BindingDB Entry DOI: 10.7270/Q22Z18Z4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM364746
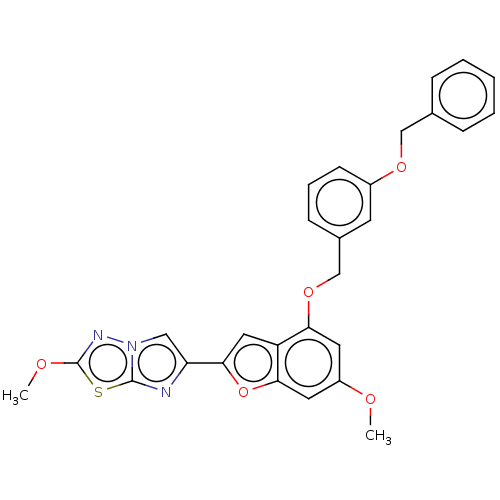
(6-(4-((3-(Benzyloxy)benzyl)oxy)-6-methoxybenzofura...)Show SMILES COc1nn2cc(nc2s1)-c1cc2c(OCc3cccc(OCc4ccccc4)c3)cc(OC)cc2o1 Show InChI InChI=1S/C28H23N3O5S/c1-32-21-12-24(35-17-19-9-6-10-20(11-19)34-16-18-7-4-3-5-8-18)22-14-26(36-25(22)13-21)23-15-31-27(29-23)37-28(30-31)33-2/h3-15H,16-17H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 in human liver microsomes |
J Med Chem 62: 7400-7416 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00186
BindingDB Entry DOI: 10.7270/Q2QC06ZZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50536234

(CHEMBL4580214)Show SMILES C[C@H]1CC[C@@H](CC1)C(=O)N(CC(=O)N1CCOCC1)c1cc(sc1C(O)=O)C#CC(C)(C)C |r,wU:1.0,wD:4.7,(36.38,-2.29,;35.52,-3.57,;36.19,-4.95,;35.33,-6.22,;33.79,-6.11,;33.11,-4.74,;33.98,-3.46,;32.94,-7.39,;33.62,-8.77,;31.4,-7.29,;30.72,-5.91,;29.18,-5.81,;28.33,-7.09,;28.5,-4.43,;29.37,-3.14,;28.7,-1.78,;27.16,-1.67,;26.3,-2.94,;26.98,-4.33,;30.55,-8.57,;29.01,-8.63,;28.58,-10.11,;29.86,-10.97,;31.08,-10.02,;32.4,-10.79,;32.4,-12.33,;33.74,-10.02,;27.14,-10.64,;25.68,-11.17,;24.24,-11.7,;23.06,-10.71,;23.97,-13.22,;22.58,-12.31,)| Show InChI InChI=1S/C25H34N2O5S/c1-17-5-7-18(8-6-17)23(29)27(16-21(28)26-11-13-32-14-12-26)20-15-19(9-10-25(2,3)4)33-22(20)24(30)31/h15,17-18H,5-8,11-14,16H2,1-4H3,(H,30,31)/t17-,18- | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
J Med Chem 59: 6293-302 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00541
BindingDB Entry DOI: 10.7270/Q23T9MQ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50529361
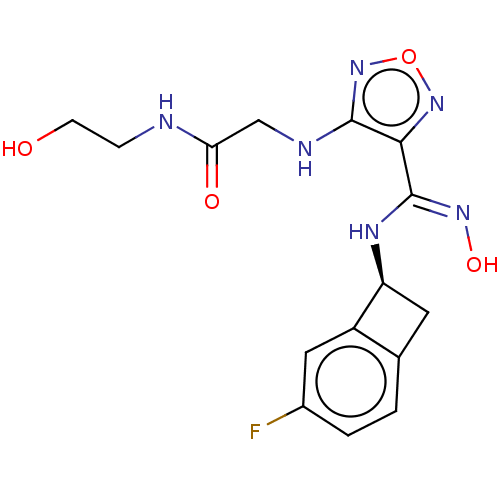
(CHEMBL4515137 | US10988487, Example 52)Show SMILES OCCNC(=O)CNc1nonc1\C(N[C@H]1Cc2ccc(F)cc12)=N\O |r| Show InChI InChI=1S/C15H17FN6O4/c16-9-2-1-8-5-11(10(8)6-9)19-15(20-25)13-14(22-26-21-13)18-7-12(24)17-3-4-23/h1-2,6,11,23,25H,3-5,7H2,(H,17,24)(H,18,22)(H,19,20)/t11-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
ACS Med Chem Lett 10: 1530-1536 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00344
BindingDB Entry DOI: 10.7270/Q22B92G6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50543291
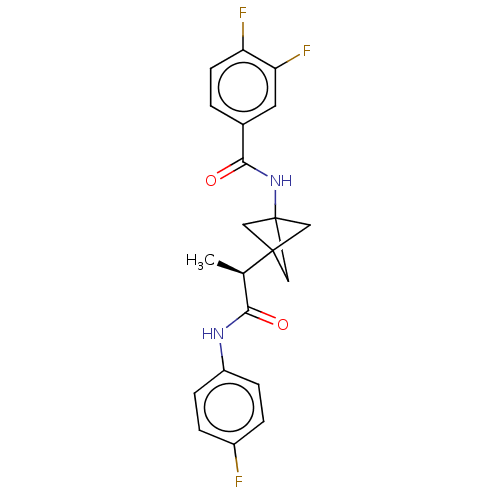
(CHEMBL4644751)Show SMILES C[C@H](C(=O)Nc1ccc(F)cc1)C12CC(C1)(C2)NC(=O)c1ccc(F)c(F)c1 |r| Show InChI InChI=1S/C21H19F3N2O2/c1-12(18(27)25-15-5-3-14(22)4-6-15)20-9-21(10-20,11-20)26-19(28)13-2-7-16(23)17(24)8-13/h2-8,12H,9-11H2,1H3,(H,25,27)(H,26,28)/t12-,20?,21?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP450 (unknown origin) |
ACS Med Chem Lett 11: 1548-1554 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00195
BindingDB Entry DOI: 10.7270/Q2RB786J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50593627
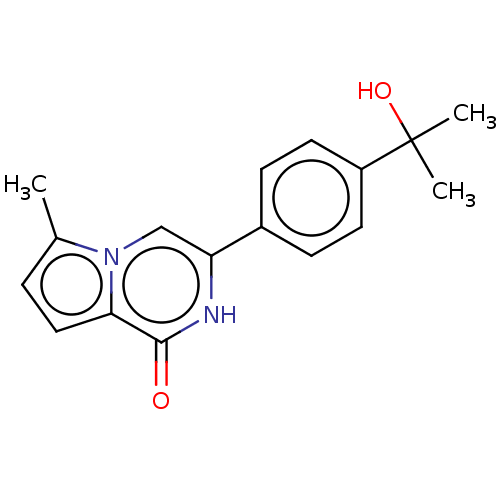
(CHEMBL5199558) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against dihydrofolate reductase (DHFR) from Escherichia coli |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50585281

(CHEMBL5079623)Show SMILES C[C@H](C(O)=O)n1nc(Cn2cc3cc(OCC4CC4)c(Cl)cc3n2)c2ccccc2c1=O |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP450 in human liver microsomes |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01066
BindingDB Entry DOI: 10.7270/Q2VM4H5J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 4B1
(Homo sapiens) | BDBM50607543
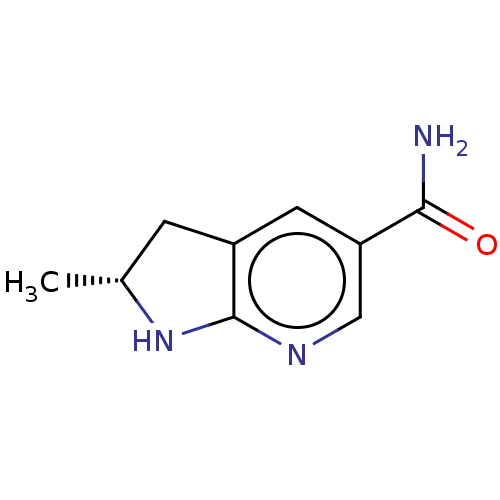
(CHEMBL5220590) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01166
BindingDB Entry DOI: 10.7270/Q2H41WHH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data