

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Canis lupus familiaris) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544931 (CHEMBL4639673) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544933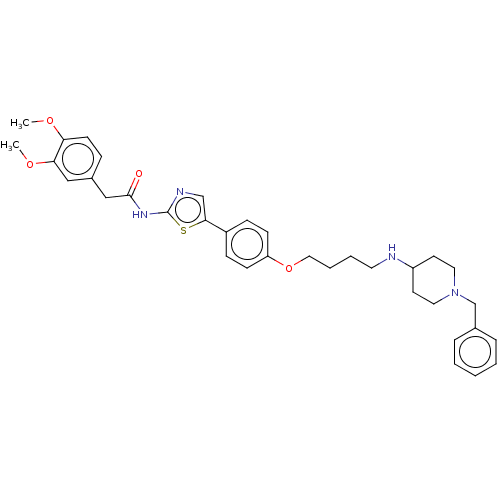 (CHEMBL4646119) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544932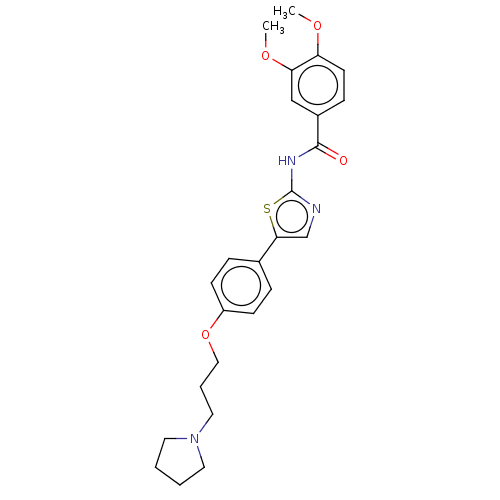 (CHEMBL4634668) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544930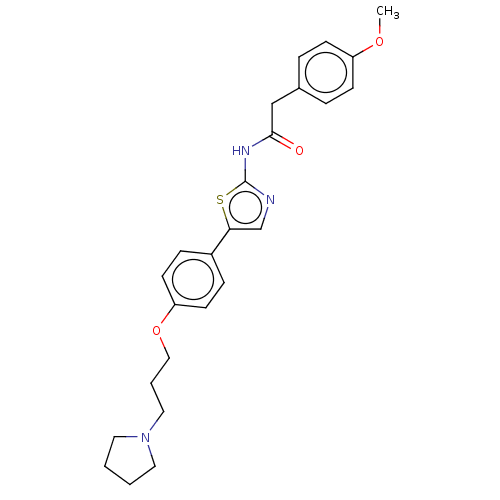 (CHEMBL4640975) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544926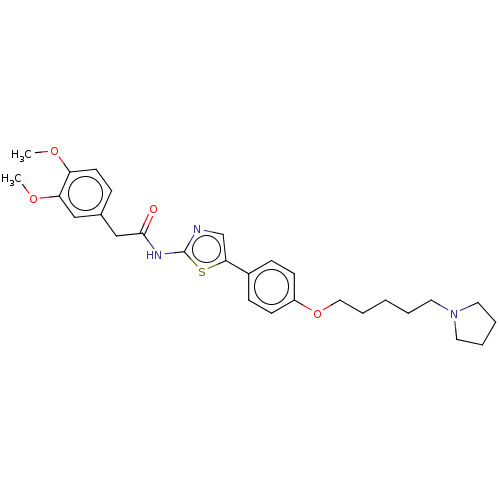 (CHEMBL4644714) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544927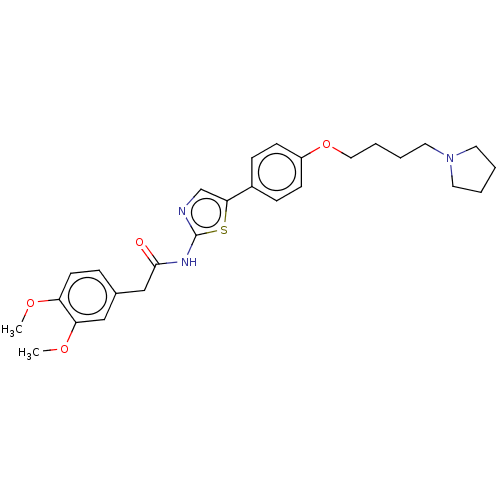 (CHEMBL4648831) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544935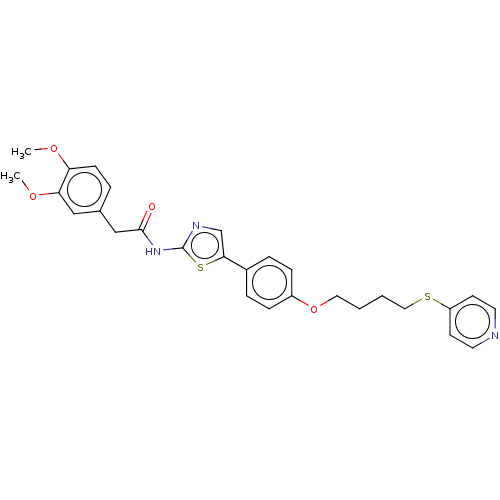 (CHEMBL4638000) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544929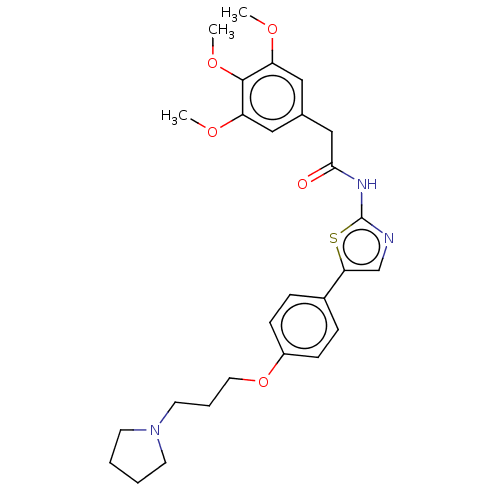 (CHEMBL4638435) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544928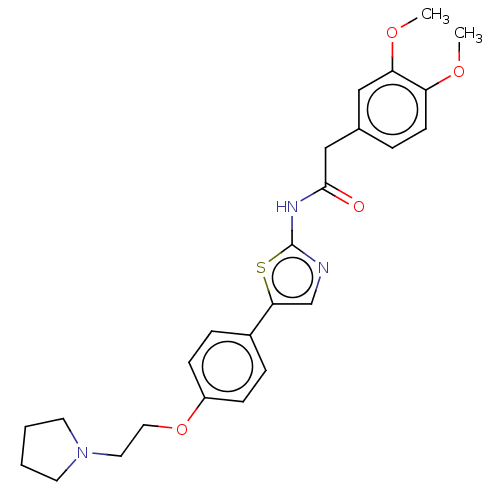 (CHEMBL4645455) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544934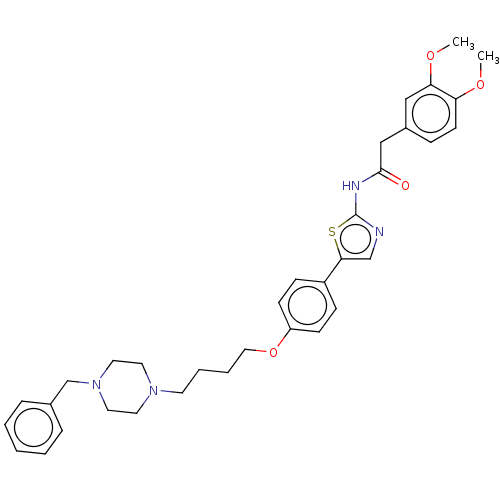 (CHEMBL4645093) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50544936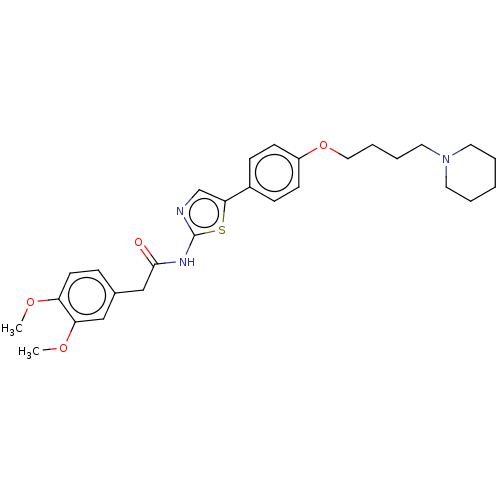 (CHEMBL4649371) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Canis lupus familiaris) | BDBM50199522 ((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei University Curated by ChEMBL | Assay Description Inhibition of dog serum BChE using butrylthiocholine iodide as substrate by spectrophotometry based Ellman's method | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126985 BindingDB Entry DOI: 10.7270/Q2QR51QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||