 Found 31 hits of ic50 for UniProtKB: P20231
Found 31 hits of ic50 for UniProtKB: P20231 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50131976
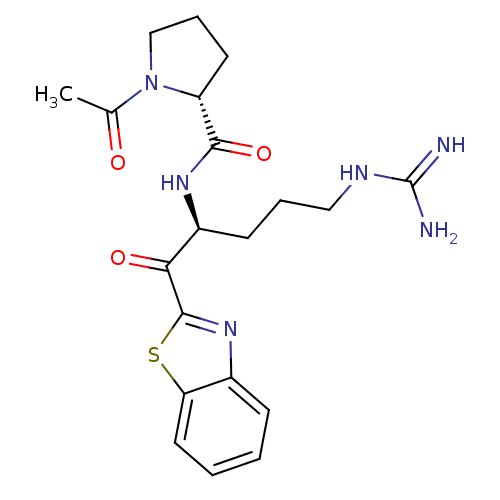
(1-Acetyl-pyrrolidine-2-carboxylic acid [1-(benzoth...)Show SMILES CC(=O)N1CCC[C@@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C20H26N6O3S/c1-12(27)26-11-5-8-15(26)18(29)24-14(7-4-10-23-20(21)22)17(28)19-25-13-6-2-3-9-16(13)30-19/h2-3,6,9,14-15H,4-5,7-8,10-11H2,1H3,(H,24,29)(H4,21,22,23)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound was determined against human Tryptase beta |
J Med Chem 46: 3865-76 (2003)
Article DOI: 10.1021/jm030050p
BindingDB Entry DOI: 10.7270/Q23T9J0V |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167514
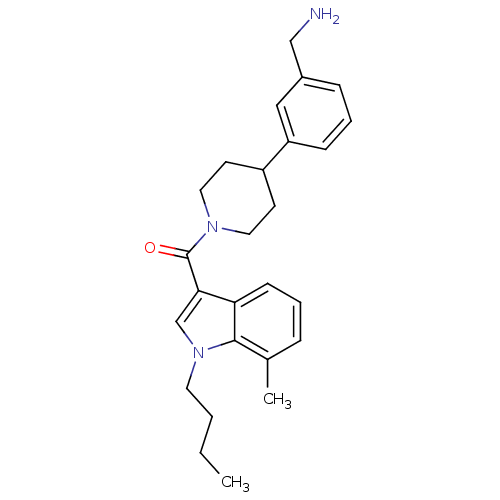
(CHEMBL370463 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2cccc(C)c12 Show InChI InChI=1S/C26H33N3O/c1-3-4-13-29-18-24(23-10-5-7-19(2)25(23)29)26(30)28-14-11-21(12-15-28)22-9-6-8-20(16-22)17-27/h5-10,16,18,21H,3-4,11-15,17,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524338
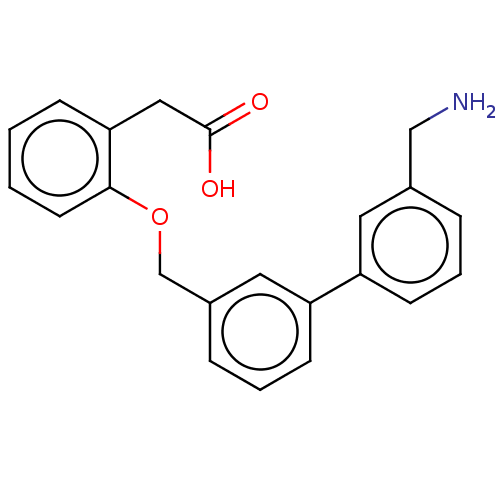
(CHEMBL4468000)Show InChI InChI=1S/C22H21NO3/c23-14-16-5-3-8-18(11-16)19-9-4-6-17(12-19)15-26-21-10-2-1-7-20(21)13-22(24)25/h1-12H,13-15,23H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162761
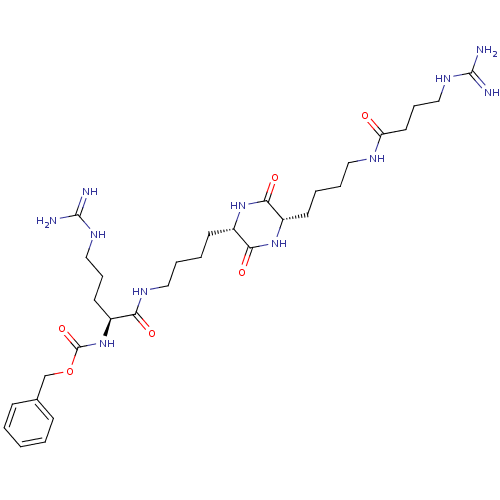
(CHEMBL180302 | [4-Guanidino-1-((S)-4-{(S)-5-[4-((S...)Show SMILES NC(=N)NCCC[C@H](NC(=O)OCc1ccccc1)C(=O)NCCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)CCCNC(N)=N)NC1=O Show InChI InChI=1S/C31H51N11O6/c32-29(33)38-18-8-14-22(42-31(47)48-20-21-10-2-1-3-11-21)26(44)37-17-7-5-13-24-28(46)40-23(27(45)41-24)12-4-6-16-36-25(43)15-9-19-39-30(34)35/h1-3,10-11,22-24H,4-9,12-20H2,(H,36,43)(H,37,44)(H,40,46)(H,41,45)(H,42,47)(H4,32,33,38)(H4,34,35,39)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524345
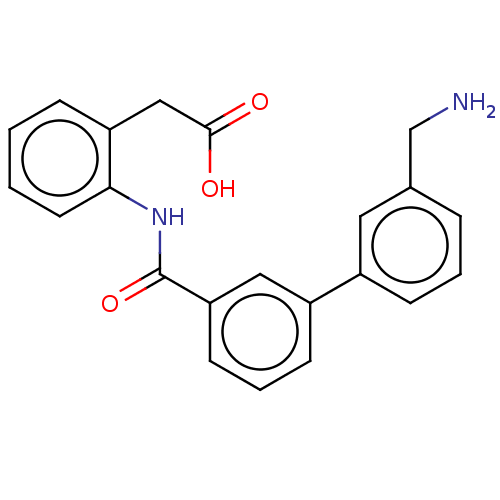
(CHEMBL4475961)Show SMILES NCc1cccc(c1)-c1cccc(c1)C(=O)Nc1ccccc1CC(O)=O Show InChI InChI=1S/C22H20N2O3/c23-14-15-5-3-7-16(11-15)17-8-4-9-19(12-17)22(27)24-20-10-2-1-6-18(20)13-21(25)26/h1-12H,13-14,23H2,(H,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162770
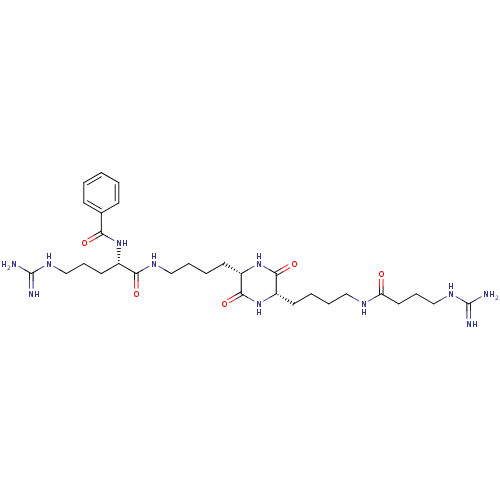
(CHEMBL180281 | N-[4-Guanidino-1-((S)-4-{(S)-5-[4-(...)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccccc1)C(=O)NCCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)CCCNC(N)=N)NC1=O Show InChI InChI=1S/C30H49N11O5/c31-29(32)37-18-8-14-21(39-25(43)20-10-2-1-3-11-20)26(44)36-17-7-5-13-23-28(46)40-22(27(45)41-23)12-4-6-16-35-24(42)15-9-19-38-30(33)34/h1-3,10-11,21-23H,4-9,12-19H2,(H,35,42)(H,36,44)(H,39,43)(H,40,46)(H,41,45)(H4,31,32,37)(H4,33,34,38)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524344
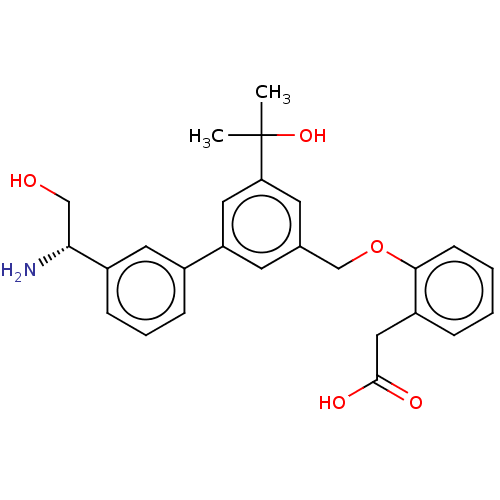
(CHEMBL4584532)Show SMILES CC(C)(O)c1cc(COc2ccccc2CC(O)=O)cc(c1)-c1cccc(c1)[C@H](N)CO |r| Show InChI InChI=1S/C26H29NO5/c1-26(2,31)22-11-17(16-32-24-9-4-3-6-20(24)14-25(29)30)10-21(13-22)18-7-5-8-19(12-18)23(27)15-28/h3-13,23,28,31H,14-16,27H2,1-2H3,(H,29,30)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524347

(CHEMBL4535197)Show SMILES N[C@H](CO)c1cccc(c1)-c1cc(Br)cc(COc2ccccc2CC(O)=O)c1 |r| Show InChI InChI=1S/C23H22BrNO4/c24-20-9-15(14-29-22-7-2-1-4-18(22)12-23(27)28)8-19(11-20)16-5-3-6-17(10-16)21(25)13-26/h1-11,21,26H,12-14,25H2,(H,27,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524340

(CHEMBL4483713)Show SMILES COCc1cc(COc2ccccc2CC(O)=O)cc(c1)-c1cccc(c1)[C@H](N)CO |r| Show InChI InChI=1S/C25H27NO5/c1-30-15-17-9-18(16-31-24-8-3-2-5-21(24)13-25(28)29)11-22(10-17)19-6-4-7-20(12-19)23(26)14-27/h2-12,23,27H,13-16,26H2,1H3,(H,28,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167514

(CHEMBL370463 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2cccc(C)c12 Show InChI InChI=1S/C26H33N3O/c1-3-4-13-29-18-24(23-10-5-7-19(2)25(23)29)26(30)28-14-11-21(12-15-28)22-9-6-8-20(16-22)17-27/h5-10,16,18,21H,3-4,11-15,17,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against alpha tryptase was determined |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50462108

(CHEMBL4246585)Show SMILES N[C@H](CO)c1cccc(c1)-c1cc(CO)cc(COc2ccccc2CC(O)=O)c1 |r| Show InChI InChI=1S/C24H25NO5/c25-22(14-27)19-6-3-5-18(11-19)21-9-16(13-26)8-17(10-21)15-30-23-7-2-1-4-20(23)12-24(28)29/h1-11,22,26-27H,12-15,25H2,(H,28,29)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162764

(CHEMBL359680 | [1-((S)-4-{(S)-5-[4-((S)-(S)-2-Benz...)Show SMILES NC(=N)NCCC[C@H](NC(=O)OCc1ccccc1)C(=O)NCCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)OCc2ccccc2)NC1=O Show InChI InChI=1S/C40H60N12O8/c41-37(42)47-23-11-19-29(51-39(57)59-25-27-13-3-1-4-14-27)33(53)45-21-9-7-17-31-35(55)50-32(36(56)49-31)18-8-10-22-46-34(54)30(20-12-24-48-38(43)44)52-40(58)60-26-28-15-5-2-6-16-28/h1-6,13-16,29-32H,7-12,17-26H2,(H,45,53)(H,46,54)(H,49,56)(H,50,55)(H,51,57)(H,52,58)(H4,41,42,47)(H4,43,44,48)/t29-,30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162760
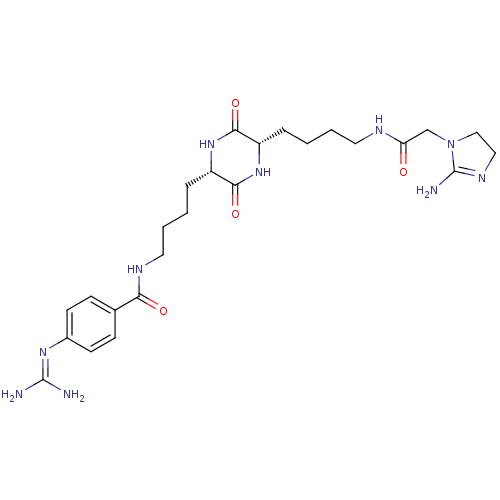
(4-Guanidino-N-[(S)-4-((S)-5-{4-[2-(2-imino-imidazo...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-[#7]-2-[#6]-[#6]-[#7]=[#6]-2-[#7])-[#7]-[#6]-1=O |c:34| Show InChI InChI=1S/C25H38N10O4/c26-24(27)32-17-9-7-16(8-10-17)21(37)30-12-4-2-6-19-23(39)33-18(22(38)34-19)5-1-3-11-29-20(36)15-35-14-13-31-25(35)28/h7-10,18-19H,1-6,11-15H2,(H2,28,31)(H,29,36)(H,30,37)(H,33,39)(H,34,38)(H4,26,27,32)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162758
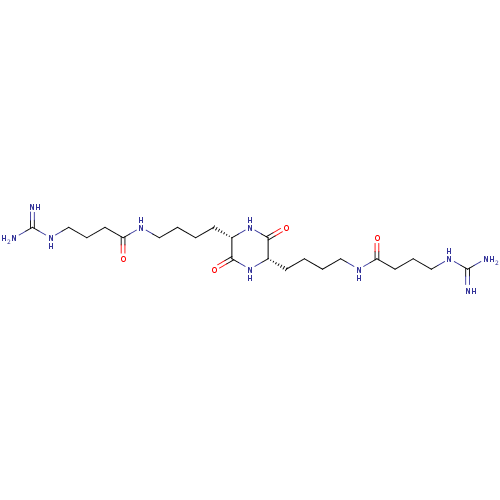
(4-Guanidino-N-(4-{(S)-5-[4-((S)-4-guanidino-butyry...)Show SMILES NC(=N)NCCCC(=O)NCCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)CCCNC(N)=N)NC1=O Show InChI InChI=1S/C22H42N10O4/c23-21(24)29-13-5-9-17(33)27-11-3-1-7-15-19(35)32-16(20(36)31-15)8-2-4-12-28-18(34)10-6-14-30-22(25)26/h15-16H,1-14H2,(H,27,33)(H,28,34)(H,31,36)(H,32,35)(H4,23,24,29)(H4,25,26,30)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162762

(CHEMBL181327 | [(S)-4-Guanidino-1-((S)-4-{(S)-5-[4...)Show SMILES NC(=N)NCCCCC(=O)NCCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)OCc2ccccc2)NC1=O Show InChI InChI=1S/C32H53N11O6/c33-30(34)39-19-9-6-16-26(44)37-17-7-4-13-24-28(46)42-25(29(47)41-24)14-5-8-18-38-27(45)23(15-10-20-40-31(35)36)43-32(48)49-21-22-11-2-1-3-12-22/h1-3,11-12,23-25H,4-10,13-21H2,(H,37,44)(H,38,45)(H,41,47)(H,42,46)(H,43,48)(H4,33,34,39)(H4,35,36,40)/t23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162763

(CHEMBL181685 | N-((S)-4-{5-[4-(3-Guanidino-propion...)Show SMILES CN(CCC(=O)NCCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)CCNC(N)=N)NC1=O)C(N)=N Show InChI InChI=1S/C21H40N10O4/c1-31(21(24)25)13-9-17(33)27-11-5-3-7-15-19(35)29-14(18(34)30-15)6-2-4-10-26-16(32)8-12-28-20(22)23/h14-15H,2-13H2,1H3,(H3,24,25)(H,26,32)(H,27,33)(H,29,35)(H,30,34)(H4,22,23,28)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524346

(CHEMBL4548070)Show SMILES N[C@H](CO)c1cccc(c1)-c1cccc(COc2ccccc2CC(O)=O)c1 |r| Show InChI InChI=1S/C23H23NO4/c24-21(14-25)19-9-4-8-18(12-19)17-7-3-5-16(11-17)15-28-22-10-2-1-6-20(22)13-23(26)27/h1-12,21,25H,13-15,24H2,(H,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524339

(CHEMBL4463116)Show SMILES CC(C)Nc1cc(COc2ccccc2CC(O)=O)cc(c1)-c1cccc(c1)[C@H](N)CO |r| Show InChI InChI=1S/C26H30N2O4/c1-17(2)28-23-11-18(16-32-25-9-4-3-6-21(25)14-26(30)31)10-22(13-23)19-7-5-8-20(12-19)24(27)15-29/h3-13,17,24,28-29H,14-16,27H2,1-2H3,(H,30,31)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162759
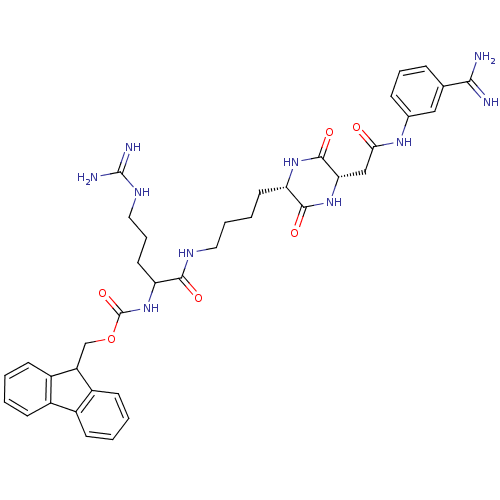
(CHEMBL178315 | [1-(4-{(S)-5-[((S)-3-Carbamimidoyl-...)Show SMILES NC(=N)NCCCC(NC(=O)OCC1c2ccccc2-c2ccccc12)C(=O)NCCCC[C@@H]1NC(=O)[C@H](CC(=O)Nc2cccc(c2)C(N)=N)NC1=O Show InChI InChI=1S/C38H46N10O6/c39-33(40)22-9-7-10-23(19-22)45-32(49)20-31-36(52)46-30(35(51)47-31)15-5-6-17-43-34(50)29(16-8-18-44-37(41)42)48-38(53)54-21-28-26-13-3-1-11-24(26)25-12-2-4-14-27(25)28/h1-4,7,9-14,19,28-31H,5-6,8,15-18,20-21H2,(H3,39,40)(H,43,50)(H,45,49)(H,46,52)(H,47,51)(H,48,53)(H4,41,42,44)/t29?,30-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162765

((S)-N-[4-Guanidino-1-((S)-4-{(S)-5-[4-(5-guanidino...)Show SMILES NC(=N)NCCCCC(=O)NCCCC[C@@H]1NC(=O)[C@H](CCCCNC(=O)[C@H](CCCNC(N)=N)NC(=O)c2ccccc2)NC1=O Show InChI InChI=1S/C31H51N11O5/c32-30(33)38-19-9-6-16-25(43)36-17-7-4-13-23-28(46)42-24(29(47)41-23)14-5-8-18-37-27(45)22(15-10-20-39-31(34)35)40-26(44)21-11-2-1-3-12-21/h1-3,11-12,22-24H,4-10,13-20H2,(H,36,43)(H,37,45)(H,40,44)(H,41,47)(H,42,46)(H4,32,33,38)(H4,34,35,39)/t22-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50302107
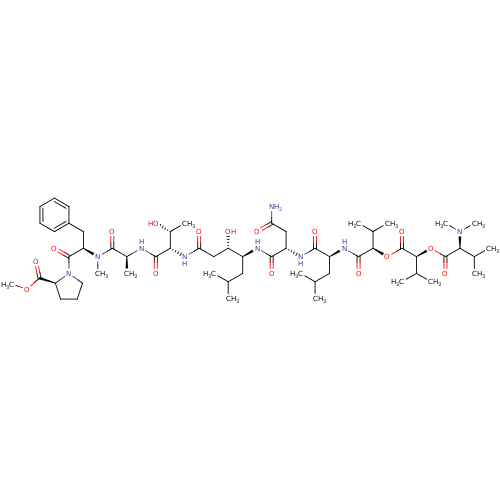
(CHEMBL567893 | Grassystatin A)Show SMILES COC(=O)[C@@H]1CCCN1C(=O)[C@@H](Cc1ccccc1)N(C)C(=O)[C@H](C)NC(=O)[C@@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](OC(=O)[C@@H](OC(=O)[C@H](C(C)C)N(C)C)C(C)C)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C58H95N9O16/c1-30(2)25-38(43(69)29-45(71)64-46(36(12)68)52(74)60-35(11)54(76)66(15)42(27-37-21-18-17-19-22-37)55(77)67-24-20-23-41(67)56(78)81-16)61-51(73)40(28-44(59)70)62-50(72)39(26-31(3)4)63-53(75)48(33(7)8)82-58(80)49(34(9)10)83-57(79)47(32(5)6)65(13)14/h17-19,21-22,30-36,38-43,46-49,68-69H,20,23-29H2,1-16H3,(H2,59,70)(H,60,74)(H,61,73)(H,62,72)(H,63,75)(H,64,71)/t35-,36+,38-,39-,40-,41-,42+,43-,46-,47-,48+,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of tryptase beta2 after 10 to 15 mins by fluorescence assay |
J Med Chem 52: 5732-47 (2009)
Article DOI: 10.1021/jm9009394
BindingDB Entry DOI: 10.7270/Q2BG2PXP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162769

(CHEMBL361835 | N-[(S)-1-(4-{(S)-5-[((S)-3-Carbamim...)Show SMILES NC(=N)NCCC[C@H](NC(=O)c1ccccc1)C(=O)NCCCC[C@@H]1NC(=O)[C@H](CC(=O)Nc2cccc(c2)C(N)=N)NC1=O Show InChI InChI=1S/C30H40N10O5/c31-25(32)19-10-6-11-20(16-19)37-24(41)17-23-29(45)39-22(28(44)40-23)12-4-5-14-35-27(43)21(13-7-15-36-30(33)34)38-26(42)18-8-2-1-3-9-18/h1-3,6,8-11,16,21-23H,4-5,7,12-15,17H2,(H3,31,32)(H,35,43)(H,37,41)(H,38,42)(H,39,45)(H,40,44)(H4,33,34,36)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162766
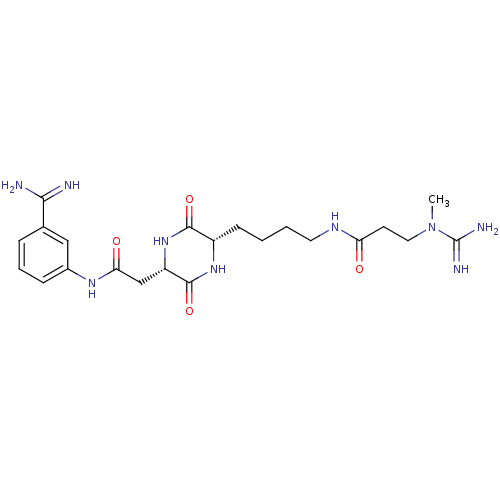
(CHEMBL181467 | N-((S)-4-{(S)-5-[(3-Carbamimidoyl-p...)Show SMILES CN(CCC(=O)NCCCC[C@@H]1NC(=O)[C@H](CC(=O)Nc2cccc(c2)C(N)=N)NC1=O)C(N)=N Show InChI InChI=1S/C22H33N9O4/c1-31(22(25)26)10-8-17(32)27-9-3-2-7-15-20(34)30-16(21(35)29-15)12-18(33)28-14-6-4-5-13(11-14)19(23)24/h4-6,11,15-16H,2-3,7-10,12H2,1H3,(H3,23,24)(H3,25,26)(H,27,32)(H,28,33)(H,29,35)(H,30,34)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162771
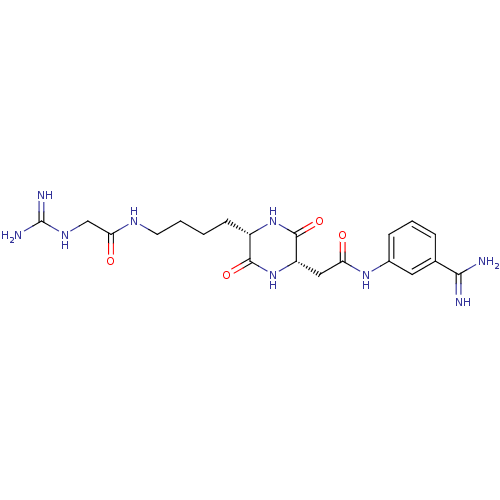
(CHEMBL181234 | N-(3-Carbamimidoyl-phenyl)-2-{(2S,5...)Show SMILES NC(=N)NCC(=O)NCCCC[C@@H]1NC(=O)[C@H](CC(=O)Nc2cccc(c2)C(N)=N)NC1=O Show InChI InChI=1S/C20H29N9O4/c21-17(22)11-4-3-5-12(8-11)27-15(30)9-14-19(33)28-13(18(32)29-14)6-1-2-7-25-16(31)10-26-20(23)24/h3-5,8,13-14H,1-2,6-7,9-10H2,(H3,21,22)(H,25,31)(H,27,30)(H,28,33)(H,29,32)(H4,23,24,26)/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524342

(CHEMBL4516604)Show SMILES C[C@@H](N)c1cccc(c1)-c1cccc(COc2ccccc2CC(O)=O)c1 |r| Show InChI InChI=1S/C23H23NO3/c1-16(24)18-8-5-10-20(13-18)19-9-4-6-17(12-19)15-27-22-11-3-2-7-21(22)14-23(25)26/h2-13,16H,14-15,24H2,1H3,(H,25,26)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162768
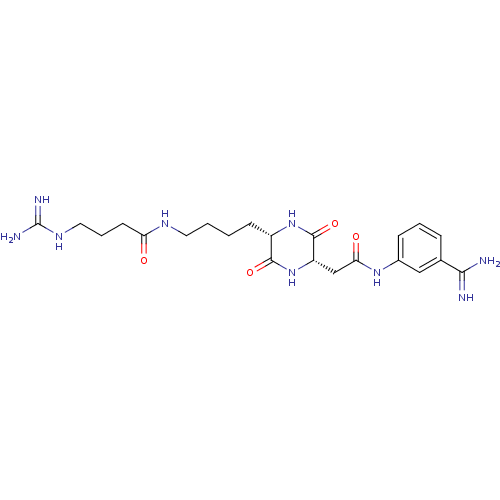
(CHEMBL178777 | N-((S)-4-{(S)-5-[(3-Carbamimidoyl-p...)Show SMILES NC(=N)NCCCC(=O)NCCCC[C@@H]1NC(=O)[C@H](CC(=O)Nc2cccc(c2)C(N)=N)NC1=O Show InChI InChI=1S/C22H33N9O4/c23-19(24)13-5-3-6-14(11-13)29-18(33)12-16-21(35)30-15(20(34)31-16)7-1-2-9-27-17(32)8-4-10-28-22(25)26/h3,5-6,11,15-16H,1-2,4,7-10,12H2,(H3,23,24)(H,27,32)(H,29,33)(H,30,35)(H,31,34)(H4,25,26,28)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50162767
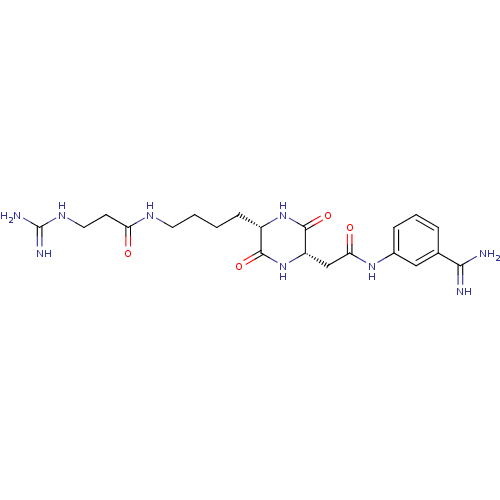
(CHEMBL180850 | N-((S)-4-{(S)-5-[(3-Carbamimidoyl-p...)Show SMILES NC(=N)NCCC(=O)NCCCC[C@@H]1NC(=O)[C@H](CC(=O)Nc2cccc(c2)C(N)=N)NC1=O Show InChI InChI=1S/C21H31N9O4/c22-18(23)12-4-3-5-13(10-12)28-17(32)11-15-20(34)29-14(19(33)30-15)6-1-2-8-26-16(31)7-9-27-21(24)25/h3-5,10,14-15H,1-2,6-9,11H2,(H3,22,23)(H,26,31)(H,28,32)(H,29,34)(H,30,33)(H4,24,25,27)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory concentration against tryptase |
Bioorg Med Chem Lett 15: 1659-64 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.048
BindingDB Entry DOI: 10.7270/Q21N80N1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50524343

(CHEMBL4546264)Show SMILES N[C@H](CCO)c1cccc(c1)-c1cccc(COc2ccccc2CC(O)=O)c1 |r| Show InChI InChI=1S/C24H25NO4/c25-22(11-12-26)20-9-4-8-19(14-20)18-7-3-5-17(13-18)16-29-23-10-2-1-6-21(23)15-24(27)28/h1-10,13-14,22,26H,11-12,15-16,25H2,(H,27,28)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of tryptase-beta2 (unknown origin) |
J Med Chem 62: 4656-4668 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00271
BindingDB Entry DOI: 10.7270/Q2CC144N |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50536839
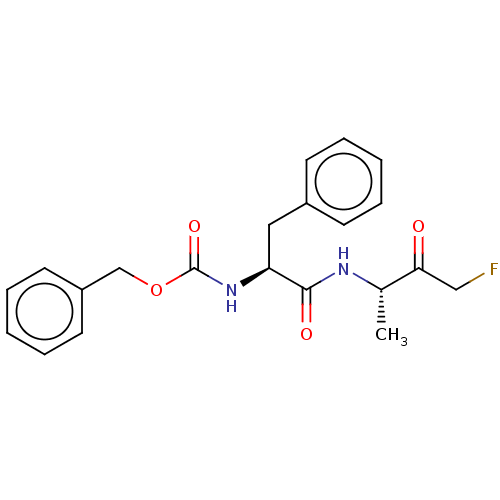
(CHEMBL2402203 | acs.jmedchem.1c00409_ST.724)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(=O)CF |r| Show InChI InChI=1S/C21H23FN2O4/c1-15(19(25)13-22)23-20(26)18(12-16-8-4-2-5-9-16)24-21(27)28-14-17-10-6-3-7-11-17/h2-11,15,18H,12-14H2,1H3,(H,23,26)(H,24,27)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of tryptase beta2 (unknown origin) using Z-GPR-AMC as substrate preincubated for 20 mins followed by substrate addition by fluorescence pl... |
ACS Med Chem Lett 7: 802-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00208
BindingDB Entry DOI: 10.7270/Q2QF8XCR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50536834
(CHEMBL4590201)Show SMILES FCC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C19H19FN2O3/c20-12-16(23)13-21-19(25)17(11-14-7-3-1-4-8-14)22-18(24)15-9-5-2-6-10-15/h1-10,17H,11-13H2,(H,21,25)(H,22,24)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of tryptase beta2 (unknown origin) using Z-GPR-AMC as substrate preincubated for 20 mins followed by substrate addition by fluorescence pl... |
ACS Med Chem Lett 7: 802-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00208
BindingDB Entry DOI: 10.7270/Q2QF8XCR |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50536838

(CHEMBL4520267)Show SMILES FCC(=O)CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C23H21FN2O3/c24-14-18(27)15-25-23(29)21(13-16-7-2-1-3-8-16)26-22(28)20-12-6-10-17-9-4-5-11-19(17)20/h1-12,21H,13-15H2,(H,25,29)(H,26,28)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development
Curated by ChEMBL
| Assay Description
Inhibition of tryptase beta2 (unknown origin) using Z-GPR-AMC as substrate preincubated for 20 mins followed by substrate addition by fluorescence pl... |
ACS Med Chem Lett 7: 802-6 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00208
BindingDB Entry DOI: 10.7270/Q2QF8XCR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data