 Found 15 hits of ic50 for UniProtKB: P37238
Found 15 hits of ic50 for UniProtKB: P37238 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50591456
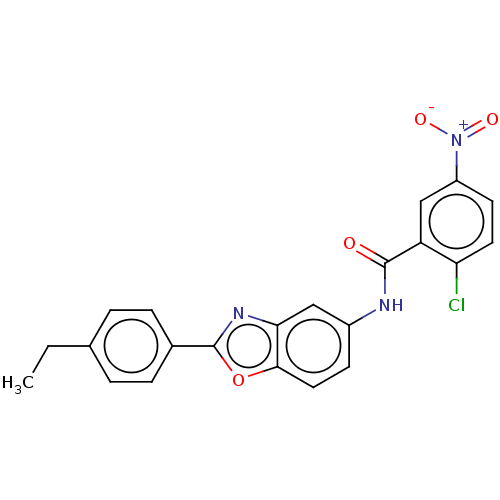
(CHEMBL5207130)Show SMILES CCc1ccc(cc1)-c1nc2cc(NC(=O)c3cc(ccc3Cl)[N+]([O-])=O)ccc2o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50610163
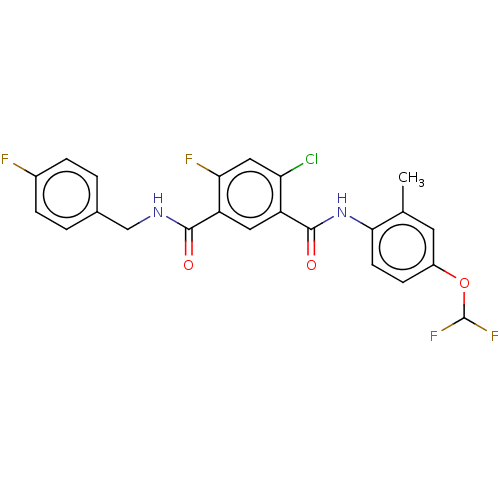
(CHEMBL5267263) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50610167
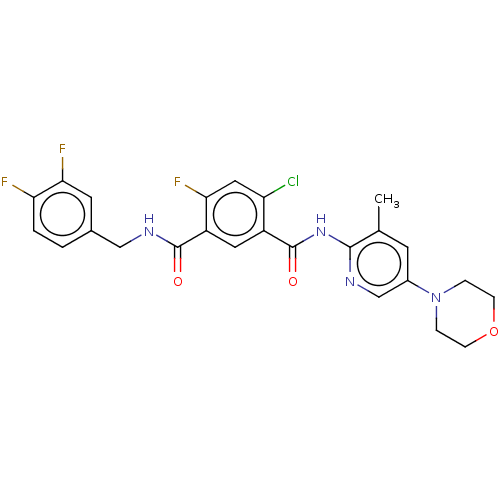
(CHEMBL5271038) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50591479
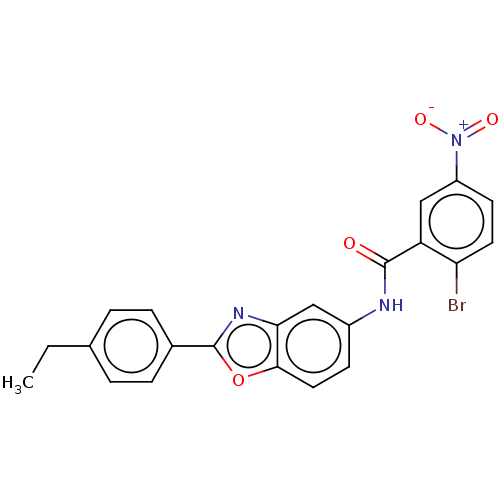
(CHEMBL5187164)Show SMILES CCc1ccc(cc1)-c1nc2cc(NC(=O)c3cc(ccc3Br)[N+]([O-])=O)ccc2o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50610162
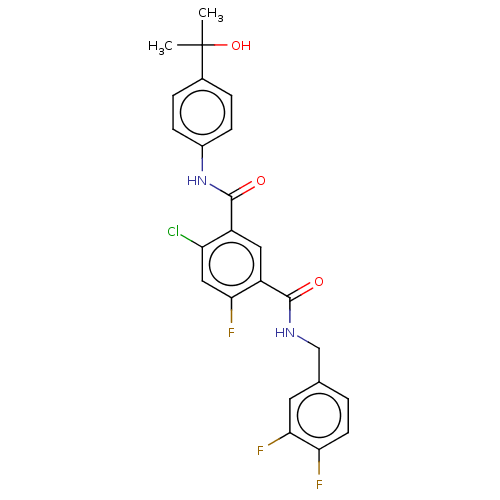
(CHEMBL5286386) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50265301

(5-(4-phenoxybenzylidene)pyrimidine-2,4,6(1H,3H,5H)...)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]\c2ccc(-[#8]-c3ccccc3)cc2)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C17H12N2O4/c20-15-14(16(21)19-17(22)18-15)10-11-6-8-13(9-7-11)23-12-4-2-1-3-5-12/h1-10H,(H2,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay |
Bioorg Med Chem Lett 18: 4959-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.028
BindingDB Entry DOI: 10.7270/Q2X066VZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50591456

(CHEMBL5207130)Show SMILES CCc1ccc(cc1)-c1nc2cc(NC(=O)c3cc(ccc3Cl)[N+]([O-])=O)ccc2o1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50265213
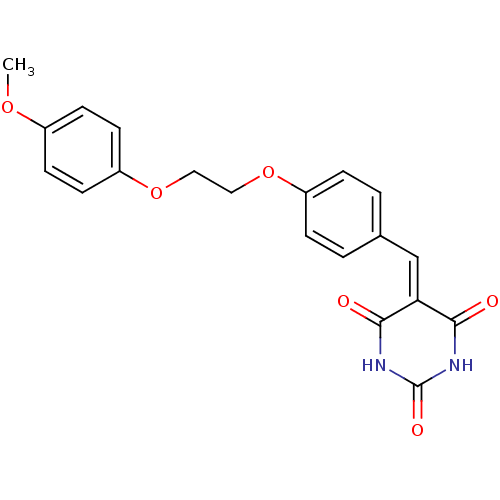
(5-(4-((4-methoxyphenoxy)methyl)benzylidene)pyrimid...)Show SMILES [#6]-[#8]-c1ccc(-[#8]-[#6]-[#6]-[#8]-c2ccc(\[#6]=[#6]-3\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-3=O)cc2)cc1 Show InChI InChI=1S/C20H18N2O6/c1-26-14-6-8-16(9-7-14)28-11-10-27-15-4-2-13(3-5-15)12-17-18(23)21-20(25)22-19(17)24/h2-9,12H,10-11H2,1H3,(H2,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay |
Bioorg Med Chem Lett 18: 4959-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.028
BindingDB Entry DOI: 10.7270/Q2X066VZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50265171
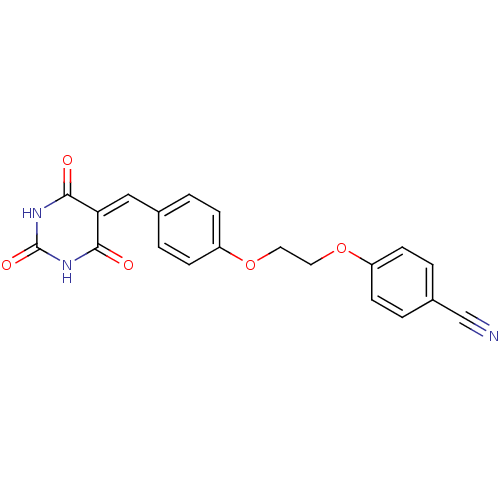
(4-(4-((2,4,6-trioxotetrahydropyrimidin-5(6H)-ylide...)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]\c2ccc(-[#8]-[#6]-[#6]-[#8]-c3ccc(cc3)C#N)cc2)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C20H15N3O5/c21-12-14-3-7-16(8-4-14)28-10-9-27-15-5-1-13(2-6-15)11-17-18(24)22-20(26)23-19(17)25/h1-8,11H,9-10H2,(H2,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay |
Bioorg Med Chem Lett 18: 4959-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.028
BindingDB Entry DOI: 10.7270/Q2X066VZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50265270

(5-(4-((10H-phenoxazin-10-yl)methyl)benzylidene)pyr...)Show SMILES O=[#6]-1-[#7]-[#6](=O)\[#6](=[#6]\c2ccc(-[#8]-[#6]-[#6]-[#7]-3-c4ccccc4-[#8]-c4ccccc-34)cc2)-[#6](=O)-[#7]-1 Show InChI InChI=1S/C25H19N3O5/c29-23-18(24(30)27-25(31)26-23)15-16-9-11-17(12-10-16)32-14-13-28-19-5-1-3-7-21(19)33-22-8-4-2-6-20(22)28/h1-12,15H,13-14H2,(H2,26,27,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay |
Bioorg Med Chem Lett 18: 4959-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.028
BindingDB Entry DOI: 10.7270/Q2X066VZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50049240
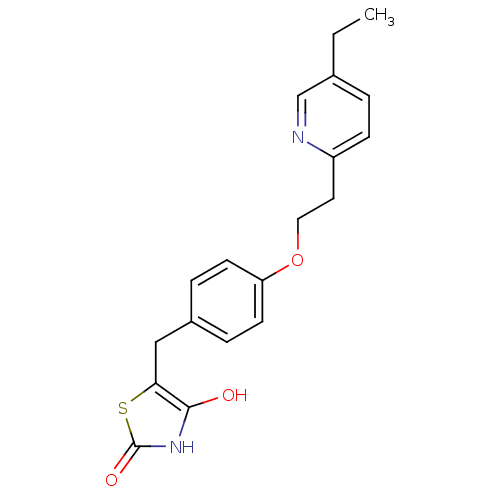
((+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)m...)Show InChI InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,22H,2,9-11H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay |
Bioorg Med Chem Lett 18: 4959-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.028
BindingDB Entry DOI: 10.7270/Q2X066VZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50265211

(5-(3-(2-(p-tolyloxy)ethoxy)benzylidene)pyrimidine-...)Show SMILES [#6]-c1ccc(-[#8]-[#6]-[#6]-[#8]-c2cccc(\[#6]=[#6]-3\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-3=O)c2)cc1 Show InChI InChI=1S/C20H18N2O5/c1-13-5-7-15(8-6-13)26-9-10-27-16-4-2-3-14(11-16)12-17-18(23)21-20(25)22-19(17)24/h2-8,11-12H,9-10H2,1H3,(H2,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay |
Bioorg Med Chem Lett 18: 4959-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.028
BindingDB Entry DOI: 10.7270/Q2X066VZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50322695
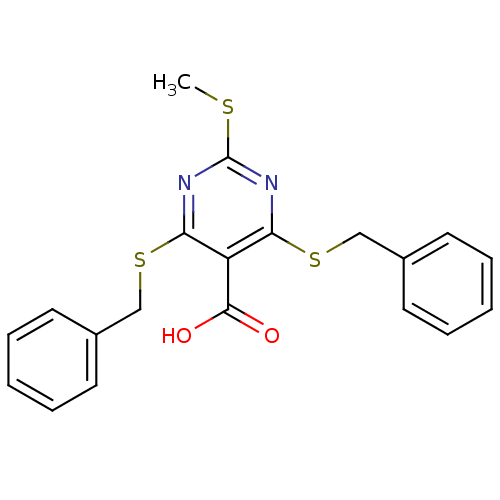
(4,6-Bis(benzylthio)-2-(methylthio)pyrimidine-5-car...)Show InChI InChI=1S/C20H18N2O2S3/c1-25-20-21-17(26-12-14-8-4-2-5-9-14)16(19(23)24)18(22-20)27-13-15-10-6-3-7-11-15/h2-11H,12-13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse PPARgamma-LBD expressed in CHO-K1 cells co-transfected with GAL4 assessed as inhibition of rosiglitazone-induced lucifer... |
J Med Chem 53: 5012-24 (2010)
Article DOI: 10.1021/jm100443s
BindingDB Entry DOI: 10.7270/Q24J0G3H |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50265212
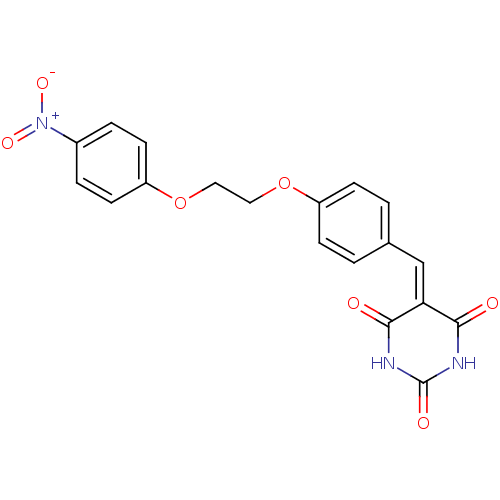
(5-(4-((4-nitrophenoxy)methyl)benzylidene)pyrimidin...)Show SMILES [#8-]-[#7+](=O)-c1ccc(-[#8]-[#6]-[#6]-[#8]-c2ccc(\[#6]=[#6]-3\[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-3=O)cc2)cc1 Show InChI InChI=1S/C19H15N3O7/c23-17-16(18(24)21-19(25)20-17)11-12-1-5-14(6-2-12)28-9-10-29-15-7-3-13(4-8-15)22(26)27/h1-8,11H,9-10H2,(H2,20,21,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from mouse PPARgamma receptor by scintillation proximation assay |
Bioorg Med Chem Lett 18: 4959-62 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.028
BindingDB Entry DOI: 10.7270/Q2X066VZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Mus musculus) | BDBM50508127
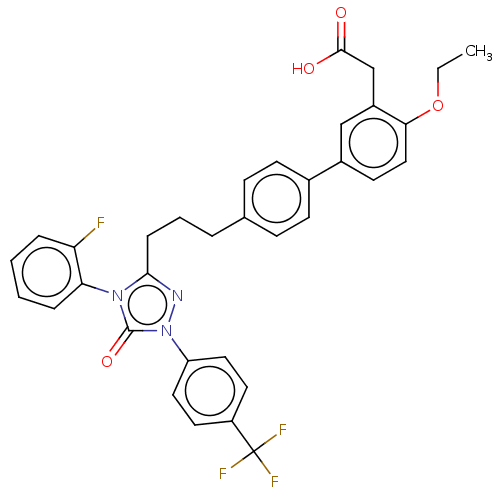
(CHEMBL4449687)Show SMILES CCOc1ccc(cc1CC(O)=O)-c1ccc(CCCc2nn(-c3ccc(cc3)C(F)(F)F)c(=O)n2-c2ccccc2F)cc1 |(50.85,-41.06,;49.52,-41.85,;48.18,-41.09,;46.86,-41.88,;45.51,-41.12,;44.19,-41.9,;44.21,-43.43,;45.54,-44.2,;46.87,-43.42,;48.21,-44.18,;48.22,-45.72,;46.9,-46.5,;49.56,-46.47,;42.88,-44.22,;42.89,-45.76,;41.57,-46.54,;40.23,-45.77,;38.9,-46.55,;37.56,-45.8,;36.23,-46.58,;34.89,-45.82,;34.41,-44.35,;32.87,-44.36,;31.96,-43.12,;32.59,-41.71,;31.68,-40.47,;30.15,-40.63,;29.53,-42.05,;30.44,-43.29,;29.24,-39.39,;29.86,-37.98,;27.71,-39.56,;28.14,-38.29,;32.4,-45.83,;30.94,-46.31,;33.65,-46.72,;33.66,-48.26,;34.99,-49.03,;35,-50.58,;33.66,-51.35,;32.33,-50.58,;32.33,-49.03,;31,-48.26,;40.21,-44.24,;41.53,-43.46,)| Show InChI InChI=1S/C34H29F4N3O4/c1-2-45-30-19-14-24(20-25(30)21-32(42)43)23-12-10-22(11-13-23)6-5-9-31-39-41(27-17-15-26(16-18-27)34(36,37)38)33(44)40(31)29-8-4-3-7-28(29)35/h3-4,7-8,10-20H,2,5-6,9,21H2,1H3,(H,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Inception Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse PPARgamma |
Bioorg Med Chem Lett 29: 503-508 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.045
BindingDB Entry DOI: 10.7270/Q2XK8JVQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data