

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50606773 (CHEMBL5220107) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114524 BindingDB Entry DOI: 10.7270/Q2PZ5DW0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556074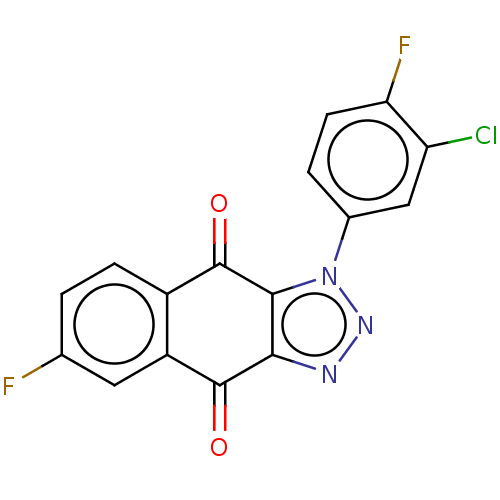 (CHEMBL4787695) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556074 (CHEMBL4787695) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114524 BindingDB Entry DOI: 10.7270/Q2PZ5DW0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50590774 (CHEMBL5203175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00303 BindingDB Entry DOI: 10.7270/Q2474G0K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556077 (CHEMBL4765090) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541856 (US11267824, Example 68a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 11.6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556061 (CHEMBL4756155) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114524 BindingDB Entry DOI: 10.7270/Q2PZ5DW0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556061 (CHEMBL4756155) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556065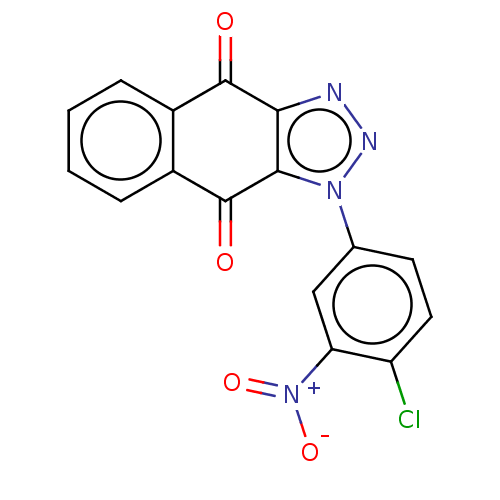 (CHEMBL4747779) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556047 (CHEMBL4780314) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541934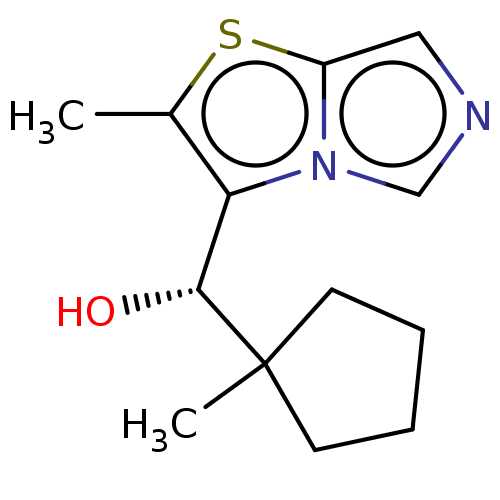 (US11267824, Example 140a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556066 (CHEMBL4791729) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556063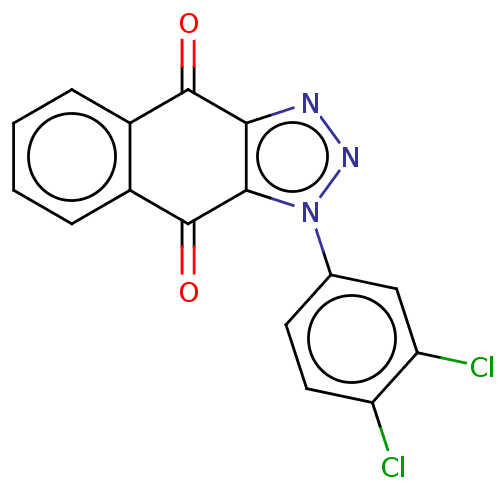 (CHEMBL4753714) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541933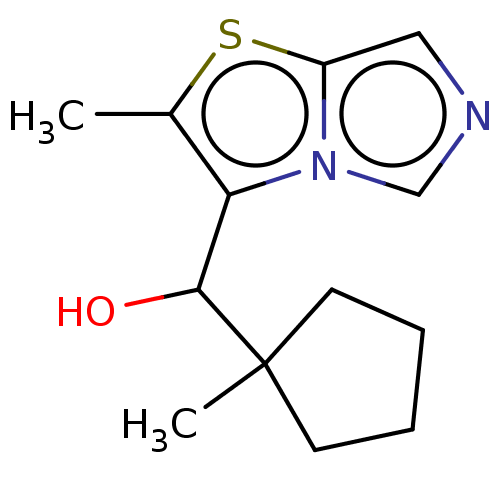 (US11267824, Example 140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50511732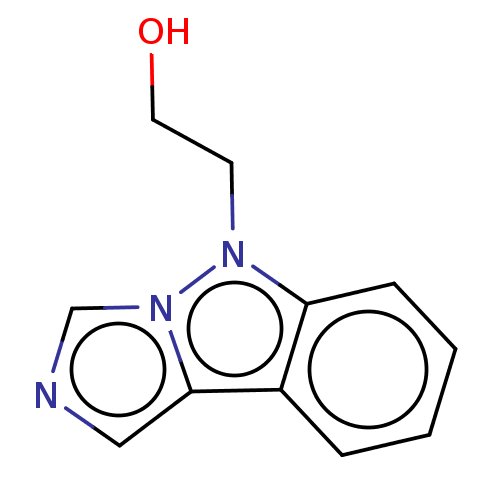 (CHEMBL4434743) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... | ACS Med Chem Lett 11: 541-549 (2020) Article DOI: 10.1021/acsmedchemlett.0c00004 BindingDB Entry DOI: 10.7270/Q2RR22JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50567017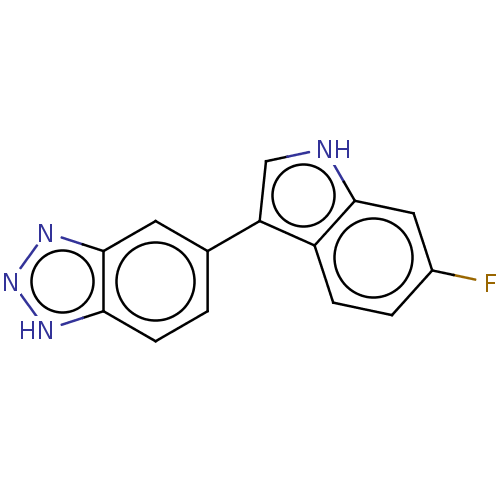 (CHEMBL4862796) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human TDO2 expressed in mouse P815B cells assessed as kynurenine concentration formation using L-tryptophan as substrate incubated for ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00323 BindingDB Entry DOI: 10.7270/Q2GM8C2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541886 (US11267824, Example 96 | US11267824, Example 96a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM370419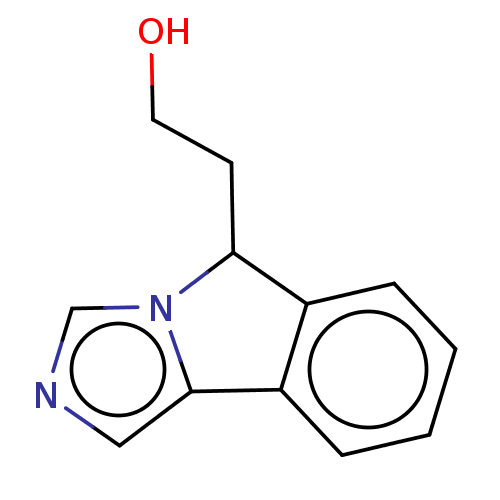 (2-(5H-imidazo[5,1-a]isoindol-5-yl)ethanol | US1023...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO using L-tryptophan as substrate preincubated for 5 mins followed by substrate addition and measured after 15 mins... | ACS Med Chem Lett 11: 541-549 (2020) Article DOI: 10.1021/acsmedchemlett.0c00004 BindingDB Entry DOI: 10.7270/Q2RR22JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556070 (CHEMBL4798028) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541834 (US11267824, Example 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50550890 (CHEMBL4747079) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO assessed as reduction in kynurenine formation using L-tryptophan as substrate incubated for 45 mins by microplate... | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111985 BindingDB Entry DOI: 10.7270/Q2W95DSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556060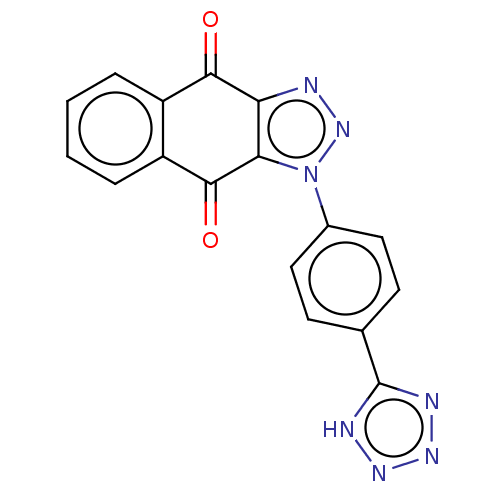 (CHEMBL4783665) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556062 (CHEMBL4785841) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50541645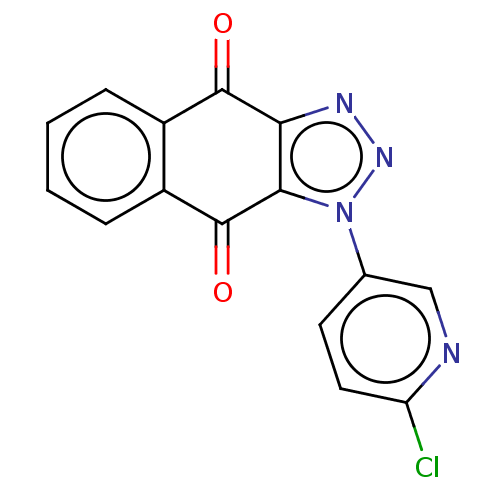 (CHEMBL4642346) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50205741 (CHEMBL3947656) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of TDO2 in human A172 cells assessed as kynurenine formation after overnight incubation | ACS Med Chem Lett 8: 11-13 (2017) Article DOI: 10.1021/acsmedchemlett.6b00458 BindingDB Entry DOI: 10.7270/Q2CZ395S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50205695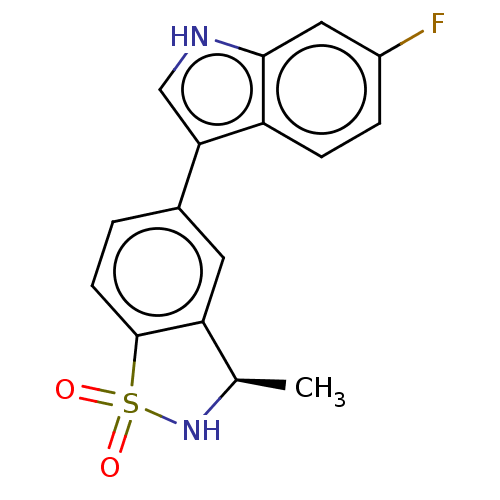 (CHEMBL3941937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of TDO2 in human A172 cells assessed as kynurenine formation after overnight incubation | ACS Med Chem Lett 8: 11-13 (2017) Article DOI: 10.1021/acsmedchemlett.6b00458 BindingDB Entry DOI: 10.7270/Q2CZ395S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50321325 (CHEMBL4173485) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO2 assessed as decrease in conversion of L-tryptophan to N-formylkynurenine preincubated for 5 mins followed by 0.2... | ACS Med Chem Lett 9: 417-421 (2018) Article DOI: 10.1021/acsmedchemlett.7b00427 BindingDB Entry DOI: 10.7270/Q2M0481G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556054 (CHEMBL4777219) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541966 (US11267824, Example 172) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50590774 (CHEMBL5203175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114625 BindingDB Entry DOI: 10.7270/Q2JW8JVD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50321332 (CHEMBL4165554) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO2 assessed as decrease in conversion of L-tryptophan to N-formylkynurenine preincubated for 5 mins followed by 0.2... | ACS Med Chem Lett 9: 417-421 (2018) Article DOI: 10.1021/acsmedchemlett.7b00427 BindingDB Entry DOI: 10.7270/Q2M0481G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50606771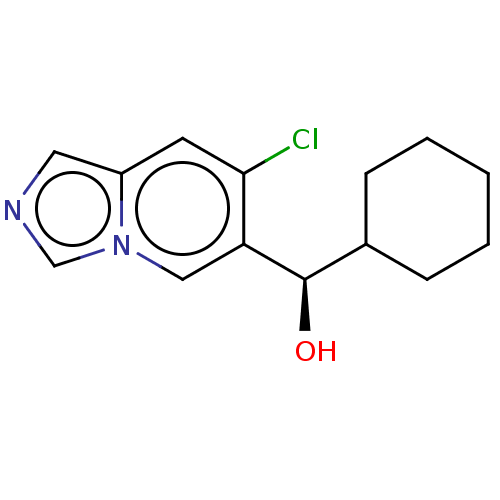 (CHEMBL5218853) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114524 BindingDB Entry DOI: 10.7270/Q2PZ5DW0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541855 (US11267824, Example 68) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 29.3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50126144 (CHEMBL3629569 | US10155972, Compound NewLink 1 | U...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human TDO2 expressed in Escherichia coli BL21 (DE3) assessed as reduction in N-formylkynurenine formation using L-tryptopha... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116160 BindingDB Entry DOI: 10.7270/Q2DF6W0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50127174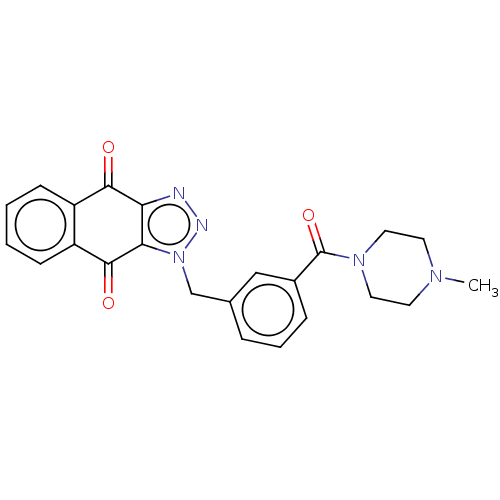 (CHEMBL3628599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO expressed in Escherichia coli incubated for 1 hr measured by fluorescence assay | J Med Chem 58: 7807-19 (2015) Article DOI: 10.1021/acs.jmedchem.5b00921 BindingDB Entry DOI: 10.7270/Q2JS9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50542388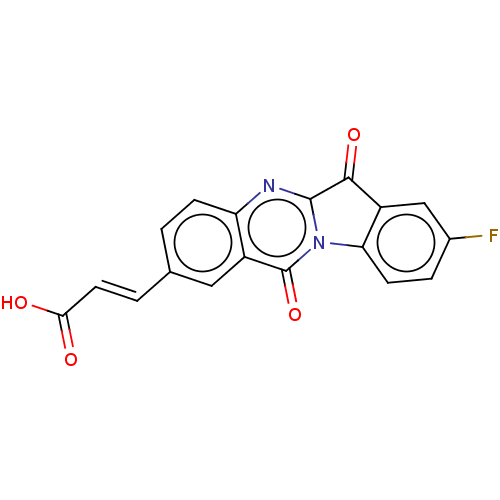 (CHEMBL4645973) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tongji University Curated by ChEMBL | Assay Description Inhibition of recombinant human TDO expressed in Escherichia coli BL21 using L-Trp as substrate incubated for 30 mins by enzymatic assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127159 BindingDB Entry DOI: 10.7270/Q2PN995R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50127174 (CHEMBL3628599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangxi Normal University Curated by ChEMBL | Assay Description Inhibition of TDO (unknown origin) | J Med Chem 63: 1544-1563 (2020) Article DOI: 10.1021/acs.jmedchem.9b01386 BindingDB Entry DOI: 10.7270/Q2J969RJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50127204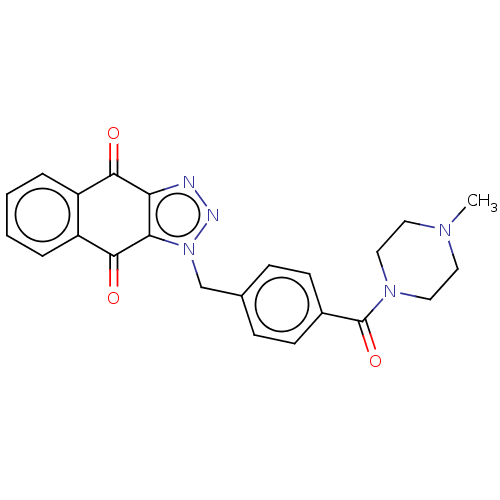 (CHEMBL3628602) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Xihua University Curated by ChEMBL | Assay Description Inhibition of TDO (unknown origin) | Bioorg Med Chem 27: 1087-1098 (2019) Article DOI: 10.1016/j.bmc.2019.02.014 BindingDB Entry DOI: 10.7270/Q2G73J3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50515069 (CHEMBL4522927 | US10899764, Example 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 30.6 | n/a | n/a | n/a | n/a | n/a | n/a |
JIANGSU HENGRUI MEDICINE CO., LTD.; SHANGHAI HENGRUI PHRMACEUTICAL CO., LTD. US Patent | Assay Description TDO: For testing, 24 μL of enzyme (TDO) was diluted 100 times with 50 mM KPB to 2400 μL. The concentration of the enzyme solution was 2.6 n... | US Patent US10899764 (2021) BindingDB Entry DOI: 10.7270/Q2MW2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556069 (CHEMBL4763934) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556064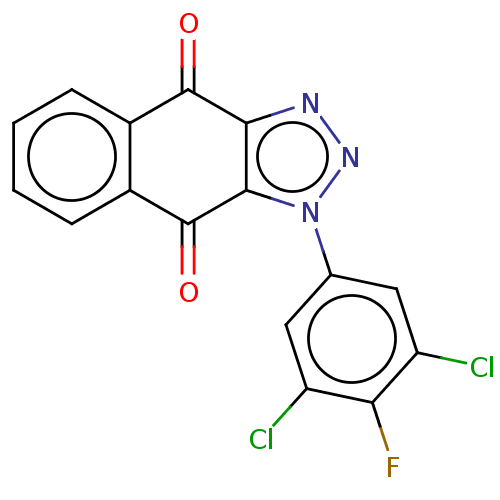 (CHEMBL4753565) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556046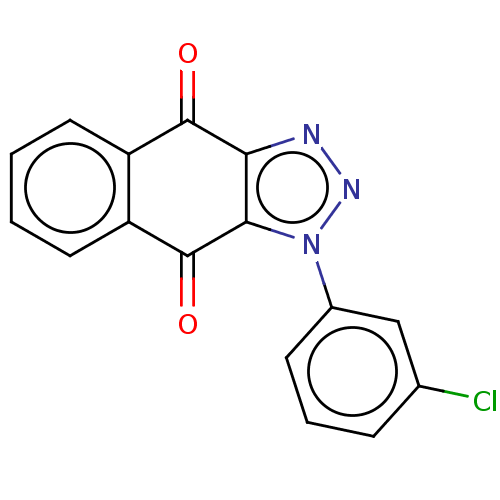 (CHEMBL4749726) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50127174 (CHEMBL3628599) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541968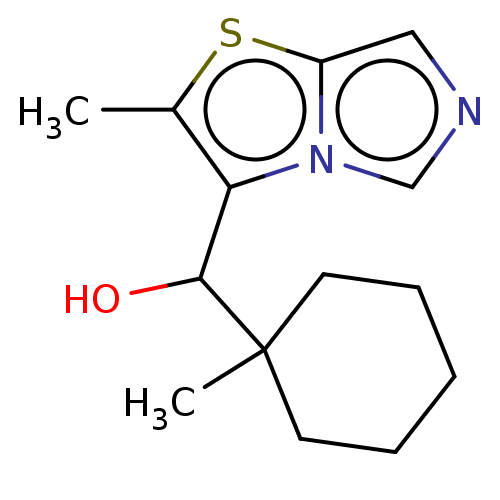 (US11267824, Example 174) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541886 (US11267824, Example 96 | US11267824, Example 96a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 39.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of TDO in human U87 MG cells using L-Trp as substrate after 8 hrs | Eur J Med Chem 160: 133-145 (2018) Article DOI: 10.1016/j.ejmech.2018.10.017 BindingDB Entry DOI: 10.7270/Q2GB27GB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50606613 (CHEMBL5219865) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00303 BindingDB Entry DOI: 10.7270/Q2474G0K | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM480515 (US10899764, Example 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
JIANGSU HENGRUI MEDICINE CO., LTD.; SHANGHAI HENGRUI PHRMACEUTICAL CO., LTD. US Patent | Assay Description TDO: For testing, 24 μL of enzyme (TDO) was diluted 100 times with 50 mM KPB to 2400 μL. The concentration of the enzyme solution was 2.6 n... | US Patent US10899764 (2021) BindingDB Entry DOI: 10.7270/Q2MW2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM50556068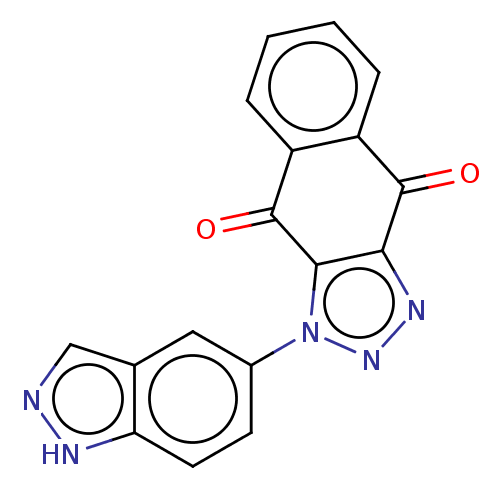 (CHEMBL4783159) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of TDO (unknown origin) assessed as reduction in L-kynurenine formation using L-tryptophan as substrate incubated for 30 mins microplate s... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112703 BindingDB Entry DOI: 10.7270/Q2639TD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 2,3-dioxygenase (Homo sapiens (Human)) | BDBM541850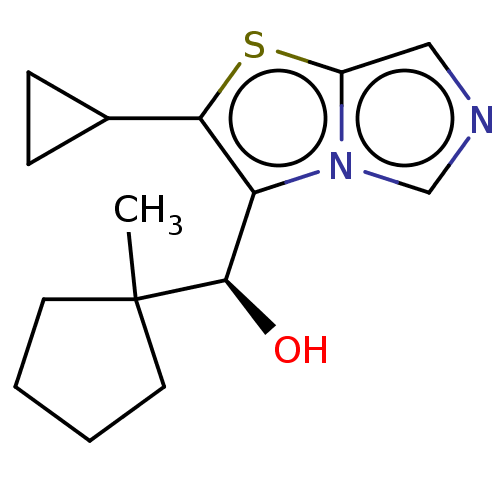 (US11267824, Example 48a) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description TDO2: Recombinant human TDO comprising amino acids 19-407 with a N-terminal hexahistidine tag expressed in E. coli and purified to homogeneity is inc... | Citation and Details BindingDB Entry DOI: 10.7270/Q2RB77T6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1324 total ) | Next | Last >> |