

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase 2 (Drosophila melanogaster) | BDBM50081427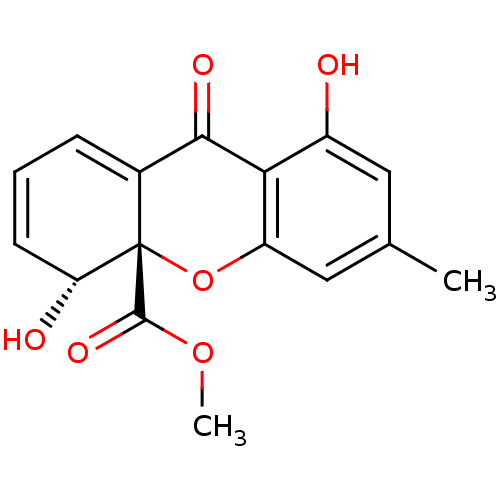 ((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay | Bioorg Med Chem Lett 9: 2653-6 (1999) BindingDB Entry DOI: 10.7270/Q2X34XZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2 (Drosophila melanogaster) | BDBM50495519 (CHEMBL3109309) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113073 BindingDB Entry DOI: 10.7270/Q2FT8R1H | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2 (Drosophila melanogaster) | BDBM50081428 ((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay | Bioorg Med Chem Lett 9: 2653-6 (1999) BindingDB Entry DOI: 10.7270/Q2X34XZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2 (Drosophila melanogaster) | BDBM50081429 ((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay | Bioorg Med Chem Lett 9: 2653-6 (1999) BindingDB Entry DOI: 10.7270/Q2X34XZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2 (Drosophila melanogaster) | BDBM50110690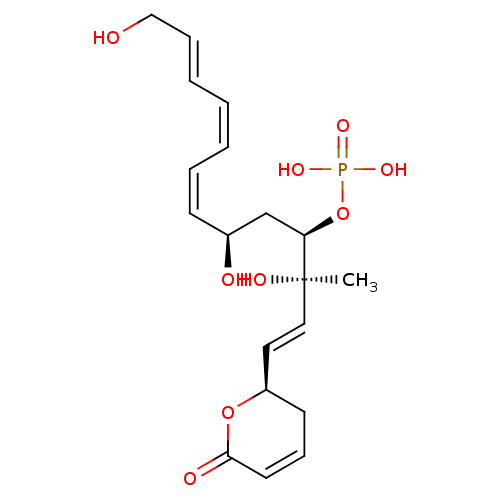 (CHEMBL17377 | FOSTRIECIN | Phosphoric acid mono-{3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Newcastle Curated by ChEMBL | Assay Description Inhibition of topoisomerase IIin vitro through a novel, non-DNA-strandcleaving mechanism | Bioorg Med Chem Lett 10: 1687-90 (2000) BindingDB Entry DOI: 10.7270/Q2GQ6Z8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2 (Drosophila melanogaster) | BDBM50081426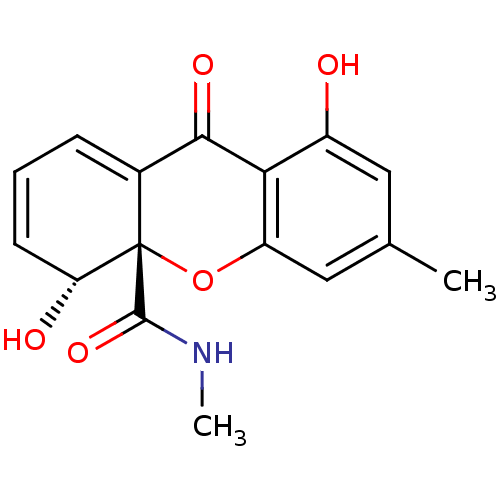 ((4R,4aS)-4,8-Dihydroxy-6-methyl-9-oxo-4,9-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay | Bioorg Med Chem Lett 9: 2653-6 (1999) BindingDB Entry DOI: 10.7270/Q2X34XZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2 (Drosophila melanogaster) | BDBM50081431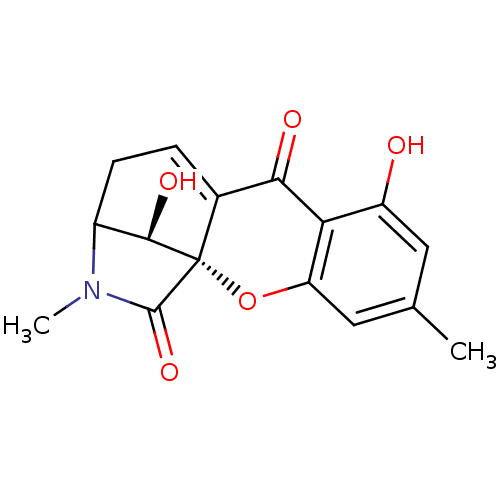 (7,16-dihydroxy-5,14-dimethyl-(1S,13S,16R)-2-oxa-14...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Co., Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against topoisomerase II (Topo II) by using decatenation assay | Bioorg Med Chem Lett 9: 2653-6 (1999) BindingDB Entry DOI: 10.7270/Q2X34XZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||