

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50129806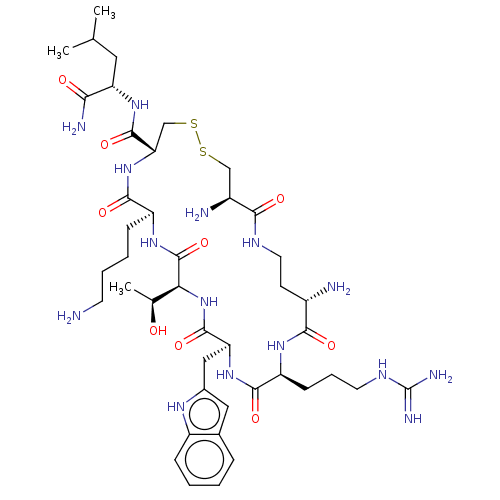 (CHEMBL3627987) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain 448 residue preincubated for 30 mins by FRET assay | Bioorg Med Chem 23: 7264-73 (2015) Article DOI: 10.1016/j.bmc.2015.10.024 BindingDB Entry DOI: 10.7270/Q2MG7RB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50129806 (CHEMBL3627987) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 30 mins and TCEP absent during reaction by FRET assay | Bioorg Med Chem 23: 7264-73 (2015) Article DOI: 10.1016/j.bmc.2015.10.024 BindingDB Entry DOI: 10.7270/Q2MG7RB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429170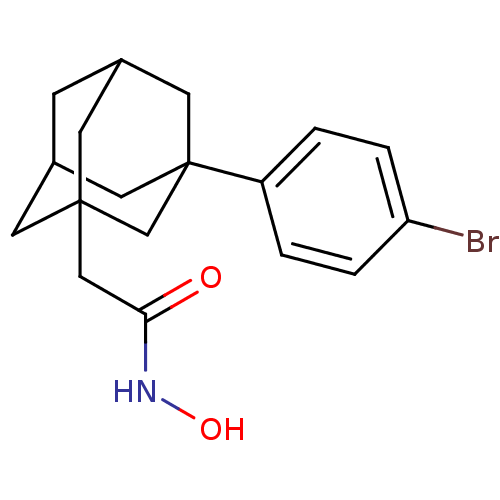 (CHEMBL2336715) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429171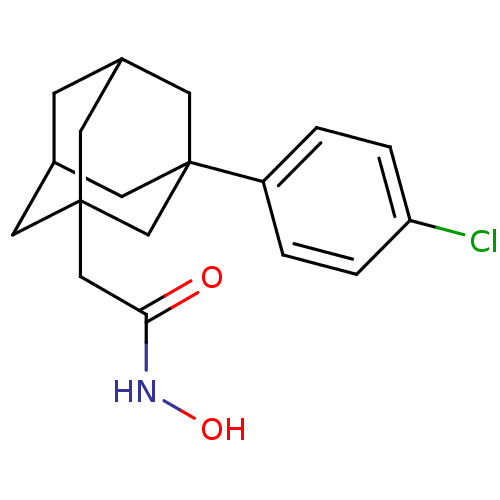 (CHEMBL2336714) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50129806 (CHEMBL3627987) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum truncated-BoNT/A light chain 424 residue preincubated for 30 mins by FRET assay | Bioorg Med Chem 23: 7264-73 (2015) Article DOI: 10.1016/j.bmc.2015.10.024 BindingDB Entry DOI: 10.7270/Q2MG7RB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50534918 (CHEMBL4474592) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of protease activity of Clostridium botulinum BoNT/A using N(K)-acetyl)-SNKTRIDEANQRATKML-carboxamide as substrate | Bioorg Med Chem 24: 4875-4889 (2016) Article DOI: 10.1016/j.bmc.2016.07.031 BindingDB Entry DOI: 10.7270/Q2JH3QPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50129806 (CHEMBL3627987) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 60 mins and TCEP absent during reaction by FRET assay | Bioorg Med Chem 23: 7264-73 (2015) Article DOI: 10.1016/j.bmc.2015.10.024 BindingDB Entry DOI: 10.7270/Q2MG7RB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50447108 (CHEMBL3112881) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A LC assessed as cleavage of SNAP-25 (141 to 206) after 30 mins by LC-MS analysis | J Med Chem 57: 669-76 (2014) Article DOI: 10.1021/jm4012164 BindingDB Entry DOI: 10.7270/Q2GH9KDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50447108 (CHEMBL3112881) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013677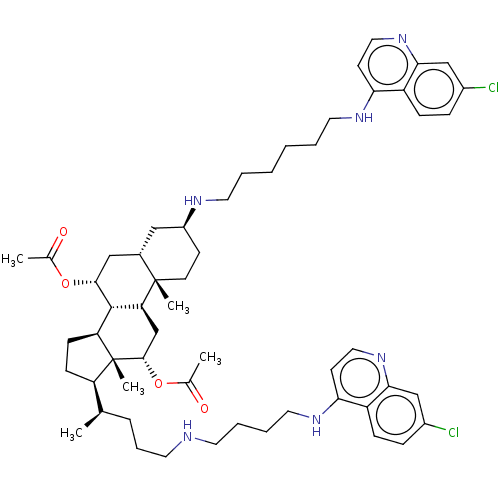 (CHEMBL3264512) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429172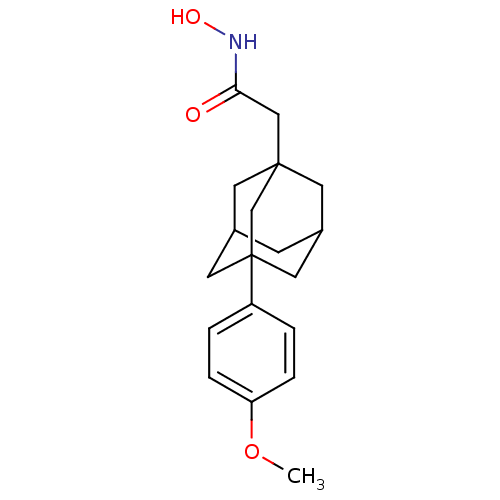 (CHEMBL2336713) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50129806 (CHEMBL3627987) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 30 mins and TCEP present during reaction by FRET assay | Bioorg Med Chem 23: 7264-73 (2015) Article DOI: 10.1016/j.bmc.2015.10.024 BindingDB Entry DOI: 10.7270/Q2MG7RB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429169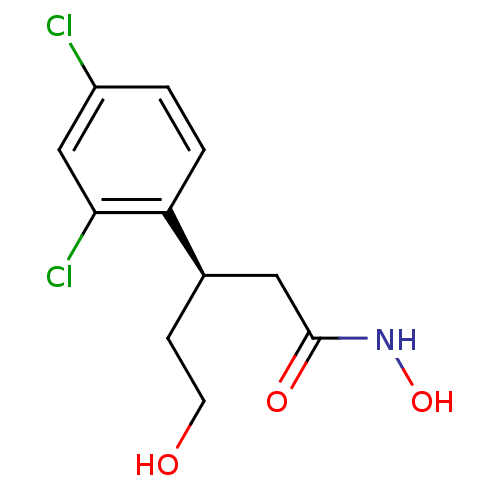 (CHEMBL2336719) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50129806 (CHEMBL3627987) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain 448 residue coincubated with TCEP for 60 mins and TCEP present during reaction by FRET assay | Bioorg Med Chem 23: 7264-73 (2015) Article DOI: 10.1016/j.bmc.2015.10.024 BindingDB Entry DOI: 10.7270/Q2MG7RB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013673 (CHEMBL3264509) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013676 (CHEMBL3264511) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013674 (CHEMBL3264510) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274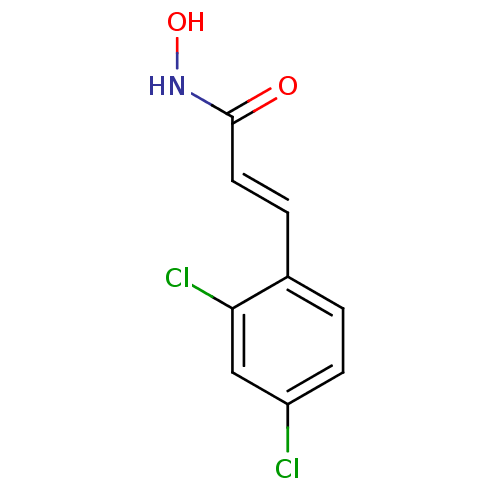 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain | Citation and Details Article DOI: 10.1039/d1md00089f BindingDB Entry DOI: 10.7270/Q24T6P2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition constant at 10 nM using SNAPtide flp6 as substrate measured after 30 mi... | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00028 BindingDB Entry DOI: 10.7270/Q2V98CX5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113877 BindingDB Entry DOI: 10.7270/Q2PK0M78 | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum truncated BoNT/A light chain (1-425) using truncated SNAP 25 (141-206) peptide as substrate by LC/MS ... | ACS Med Chem Lett 4: 283-287 (2013) Article DOI: 10.1021/ml300428s BindingDB Entry DOI: 10.7270/Q20K29WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix Inc Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum toxin BoNT/A light chain | J Med Chem 53: 2264-76 (2010) Article DOI: 10.1021/jm901852f BindingDB Entry DOI: 10.7270/Q2HQ40V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Clostridium botulinum BoNT/A using SNAP-25 (141-206) as substrate by HPLC analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01006 BindingDB Entry DOI: 10.7270/Q2WD446P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013678 (CHEMBL3264513) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384950 (CHEMBL2037386) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384950 (CHEMBL2037386) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50534917 (CHEMBL1933864) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of protease activity of Clostridium botulinum BoNT/A using N(K)-acetyl)-SNKTRIDEANQRATKML-carboxamide as substrate | Bioorg Med Chem 24: 4875-4889 (2016) Article DOI: 10.1016/j.bmc.2016.07.031 BindingDB Entry DOI: 10.7270/Q2JH3QPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50242333 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc. Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum botulinum neurotoxin type A light chain | Bioorg Med Chem 19: 7338-48 (2011) Article DOI: 10.1016/j.bmc.2011.10.062 BindingDB Entry DOI: 10.7270/Q2FF3SS3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013672 (CHEMBL3264508) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50013675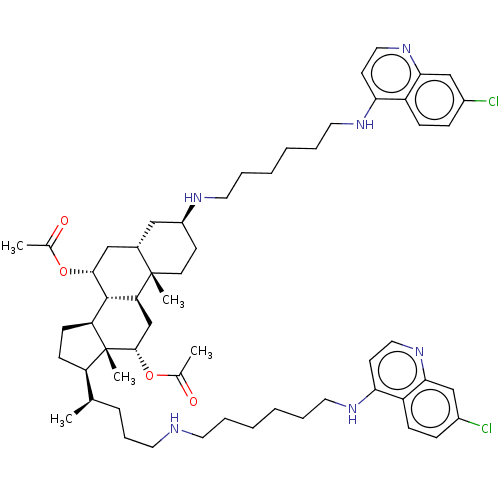 (CHEMBL3259867) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50048521 (CHEMBL3309329) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum neurotoxin serotype A light chain | Bioorg Med Chem 22: 3971-81 (2014) Article DOI: 10.1016/j.bmc.2014.06.004 BindingDB Entry DOI: 10.7270/Q20P11P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... | J Med Chem 57: 4134-53 (2014) Article DOI: 10.1021/jm500033r BindingDB Entry DOI: 10.7270/Q20Z74TB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429174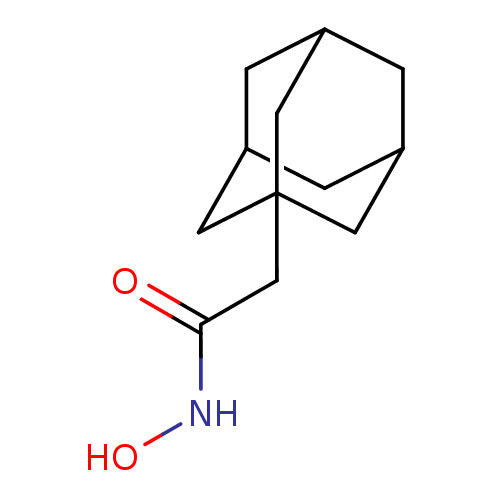 (1-Adamantyl N-Hydroxyacetamide | CHEMBL2336721) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50129807 (CHEMBL3627988) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 468 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brookhaven National Laboratory Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain 448 residue preincubated for 30 mins by FRET assay | Bioorg Med Chem 23: 7264-73 (2015) Article DOI: 10.1016/j.bmc.2015.10.024 BindingDB Entry DOI: 10.7270/Q2MG7RB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384951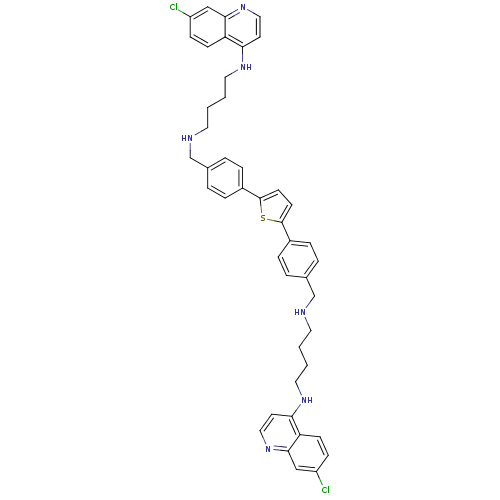 (CHEMBL2037387) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 535 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384440 (CHEMBL2035505 | CHEMBL2037389) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384440 (CHEMBL2035505 | CHEMBL2037389) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 572 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50302031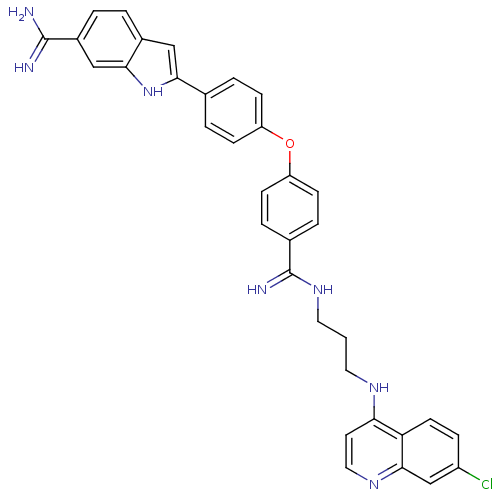 (2-(4-(4-(N'-(3-(7-chloroquinolin-4-ylamino)propyl)...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50302032 (2-(4-(4-carbamimidoylphenoxy)phenyl)-N'-(3-(7-chlo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50076267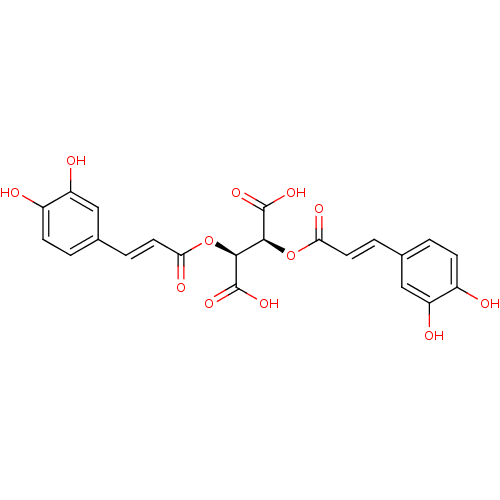 ((2S,3S)-2,3-Bis-[(E)-3-(3,4-dihydroxy-phenyl)-acry...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain using SNAP-25 as substrate by LC/MS analysis | Bioorg Med Chem 24: 4875-4889 (2016) Article DOI: 10.1016/j.bmc.2016.07.031 BindingDB Entry DOI: 10.7270/Q2JH3QPJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429173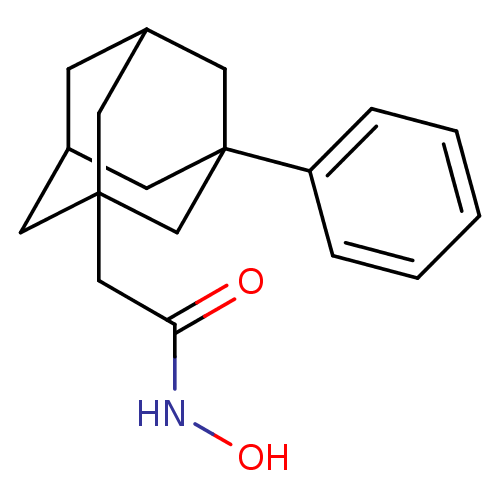 (CHEMBL2336709) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50429168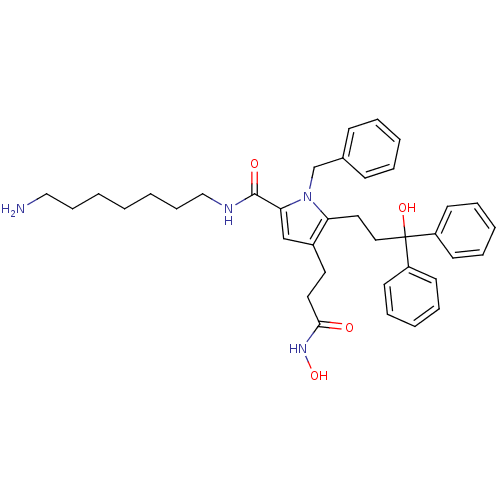 (CHEMBL2336720) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Competitive inhibition of Clostridium botulinum BoNT/A Hall A hyper protease light chain (1-425aa) using SNAP-66mer (141-206aa) as substrate by FRET ... | Bioorg Med Chem 21: 1344-8 (2013) Article DOI: 10.1016/j.bmc.2012.12.001 BindingDB Entry DOI: 10.7270/Q2N58NQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50308031 (CHEMBL591192 | N-(7-(7-aminoheptylamino)heptyl)-5-...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix Inc Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum toxin BoNT/A light chain | J Med Chem 53: 2264-76 (2010) Article DOI: 10.1021/jm901852f BindingDB Entry DOI: 10.7270/Q2HQ40V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50340404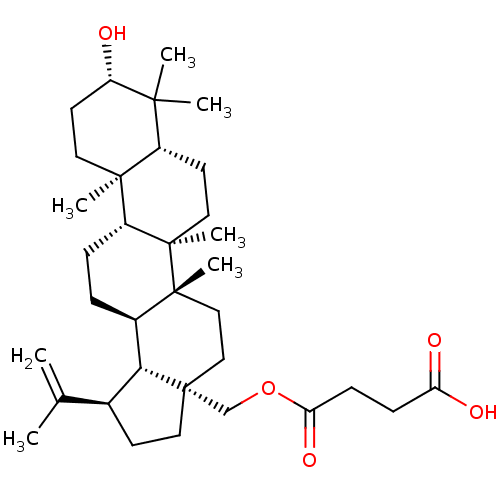 (28-Hemisuccinylbetulin | CHEMBL1761333) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium BoNT/A protease light chain | Bioorg Med Chem Lett 21: 2229-31 (2011) Article DOI: 10.1016/j.bmcl.2011.02.115 BindingDB Entry DOI: 10.7270/Q2KS6RWV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384949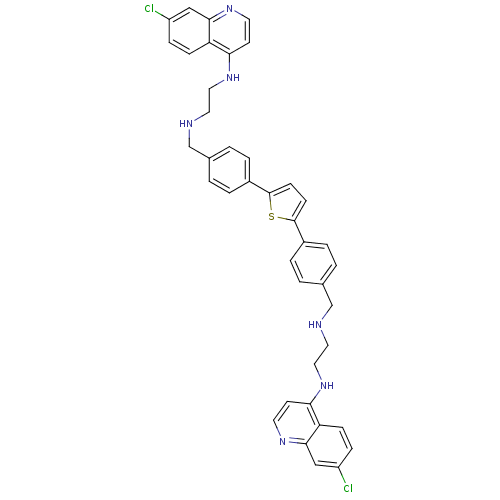 (CHEMBL2037288) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 882 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384952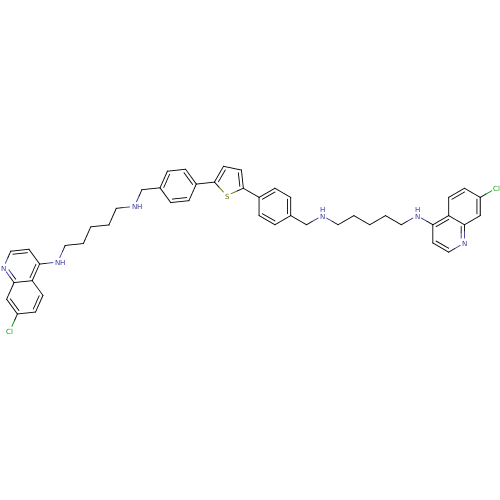 (CHEMBL2037388) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 889 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade Curated by ChEMBL | Assay Description Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysis | Eur J Med Chem 53: 374-9 (2012) Article DOI: 10.1016/j.ejmech.2012.03.043 BindingDB Entry DOI: 10.7270/Q2TB17ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50259836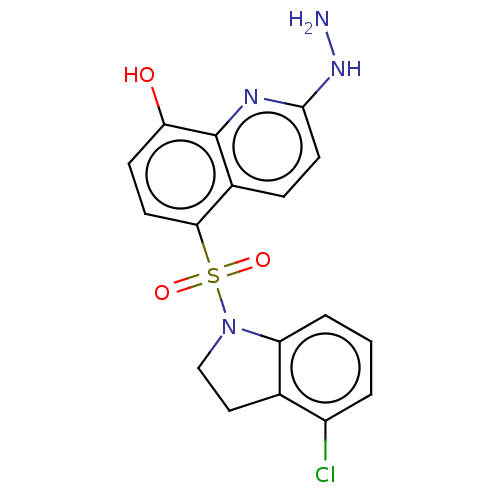 (CHEMBL4074729) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 895 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for 20 fol... | J Med Chem 60: 338-348 (2017) Article DOI: 10.1021/acs.jmedchem.6b01393 BindingDB Entry DOI: 10.7270/Q2NZ8B3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50384439 (CHEMBL2035506) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain assessed as inhibition of SNAP-25 (187-203) substrate hydrolysis by RP-HPLC-based assay in pre... | ACS Med Chem Lett 1: 301-305 (2010) Article DOI: 10.1021/ml100056v BindingDB Entry DOI: 10.7270/Q2G73FRR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50445596 (CHEMBL3103447) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum BoNT/A light chain (1 to 425 amino acids) by LC-MS analysis | Bioorg Med Chem 22: 1208-17 (2014) Article DOI: 10.1016/j.bmc.2013.11.053 BindingDB Entry DOI: 10.7270/Q2W66N71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 133 total ) | Next | Last >> |