

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 2 (Oryctolagus cuniculus) | BDBM50236292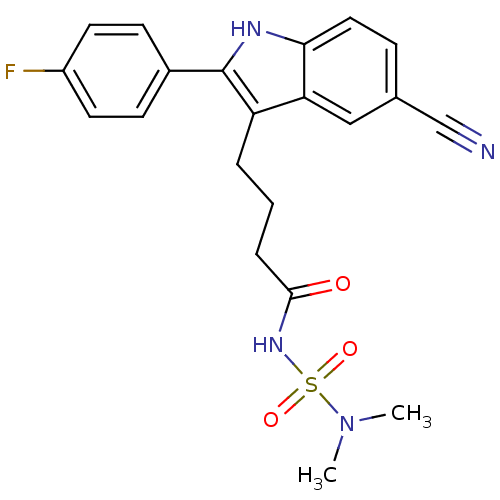 (4-[5-cyano-2-(4-fluorophenyl)-1H-indol-3-yl]-N-(di...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rabbit CXCR2 by neutrophil chemotaxis assay | Bioorg Med Chem Lett 18: 1926-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.127 BindingDB Entry DOI: 10.7270/Q20P10V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Oryctolagus cuniculus) | BDBM50236295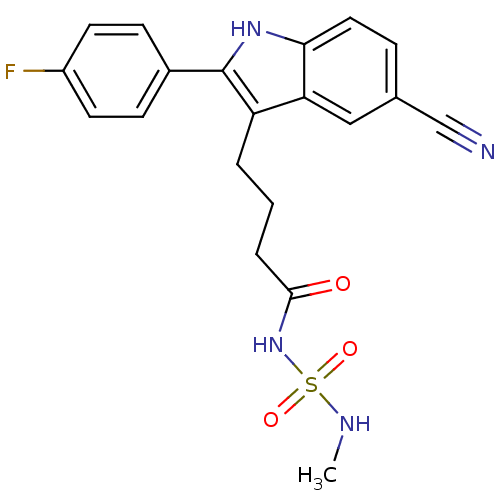 (4-[5-cyano-2-(4-fluorophenyl)-1H-indol-3-yl]-N-[(m...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rabbit CXCR2 by neutrophil chemotaxis assay | Bioorg Med Chem Lett 18: 1926-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.127 BindingDB Entry DOI: 10.7270/Q20P10V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Oryctolagus cuniculus) | BDBM50236304 (1-(2-(5-bromo-2-(4-fluorophenyl)-1H-indol-3-yl)eth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rabbit CXCR2 by neutrophil chemotaxis assay | Bioorg Med Chem Lett 18: 1926-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.127 BindingDB Entry DOI: 10.7270/Q20P10V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Oryctolagus cuniculus) | BDBM50236305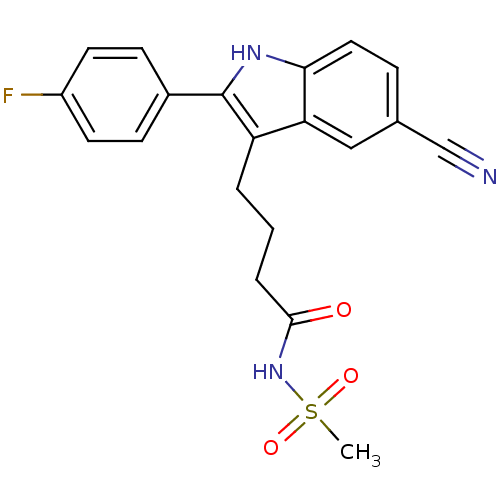 (4-(5-cyano-2-(4-fluorophenyl)-1H-indol-3-yl)-N-(me...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rabbit CXCR2 by neutrophil chemotaxis assay | Bioorg Med Chem Lett 18: 1926-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.127 BindingDB Entry DOI: 10.7270/Q20P10V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Oryctolagus cuniculus) | BDBM50236303 (4-(5-cyano-2-(4-fluorophenyl)-1H-indol-3-yl)-N-(tr...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rabbit CXCR2 by neutrophil chemotaxis assay | Bioorg Med Chem Lett 18: 1926-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.127 BindingDB Entry DOI: 10.7270/Q20P10V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Oryctolagus cuniculus) | BDBM50236296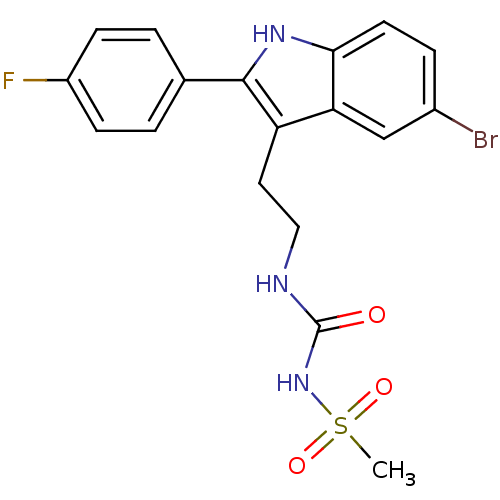 (1-(2-(5-bromo-2-(4-fluorophenyl)-1H-indol-3-yl)eth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rabbit CXCR2 by neutrophil chemotaxis assay | Bioorg Med Chem Lett 18: 1926-30 (2008) Article DOI: 10.1016/j.bmcl.2008.01.127 BindingDB Entry DOI: 10.7270/Q20P10V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||