

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332524 (4-(1-(2-chloro-6- (trifluoromethyl)benzoyl)-6- (3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332431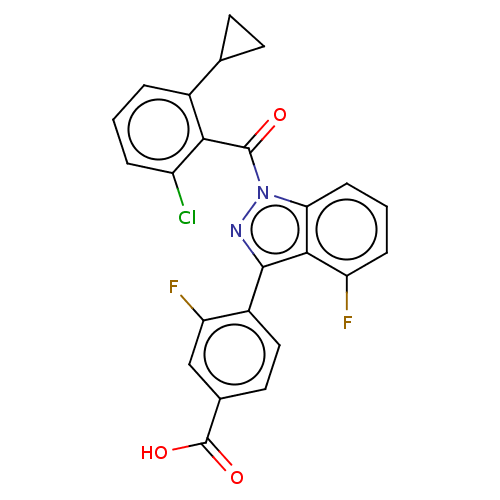 (4-(1-(2- chloro-6- cyclopropyl- benzoyl)-4- fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332523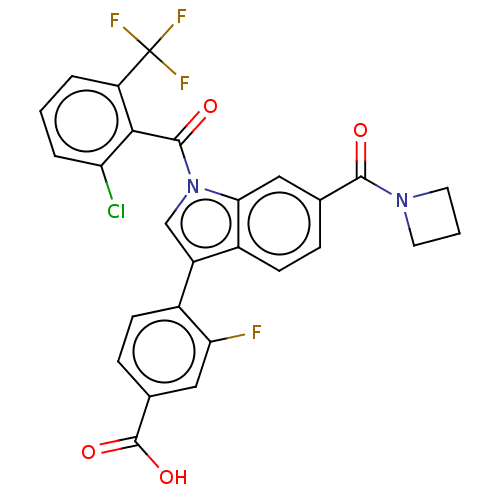 (4-(6-(azetidine-1-carbonyl)-1-(2-chloro-6-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332433 (4-(1-(2- chloro-6- cyclopropyl- benzoyl)-1H- indaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332432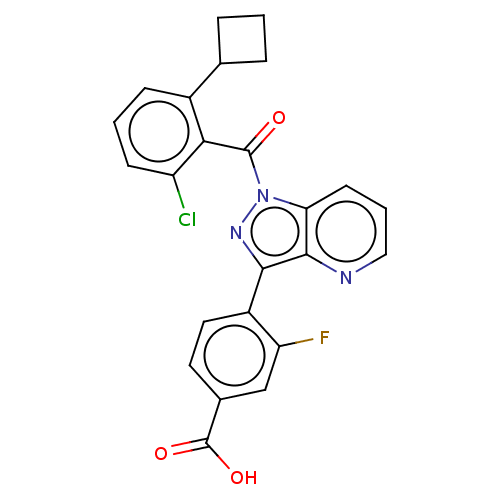 (4-(1-(2- chloro-6- cyclobutyl- benzoyl)-1H- pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332522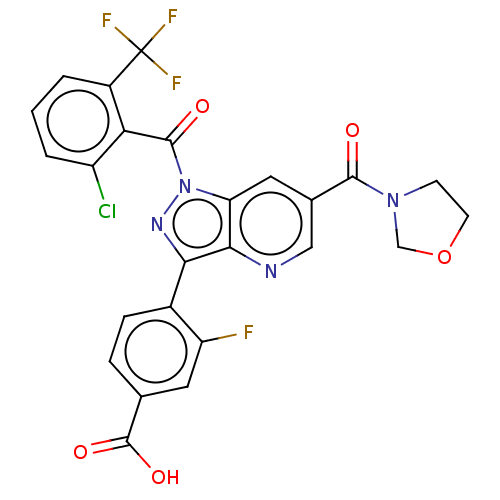 (4-(1-(2-chloro-6- (trifluoromethyl)benzoyl)- 6-(ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332520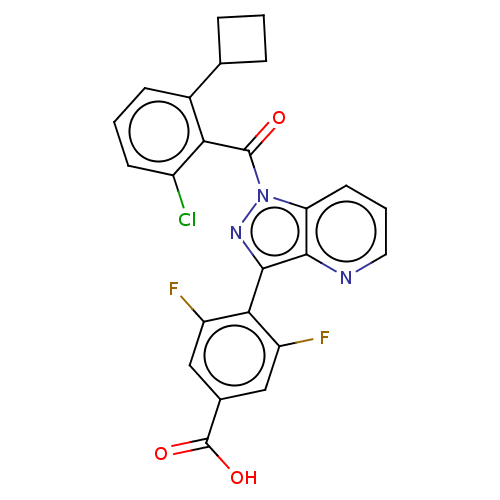 (4-(1-(2-chloro-6-cyclobutylbenzoyl)-1H-pyrazolo[4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332430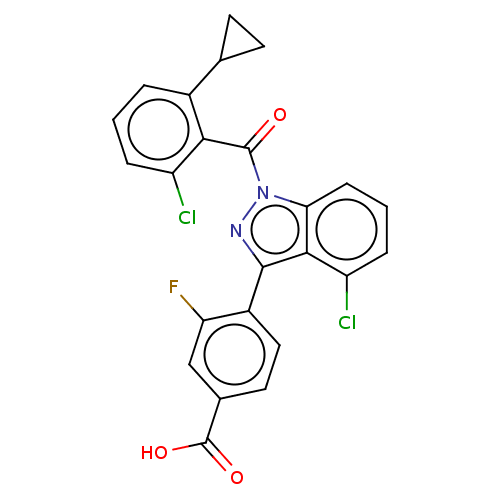 (4-(4-chloro-1- (2-chloro-6- cyclopropyl- benzoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382981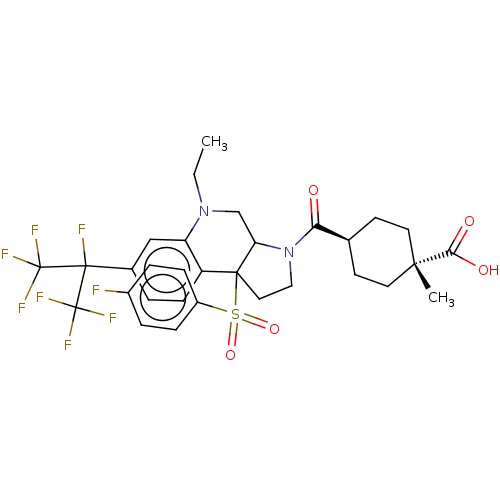 (US10273259, Example 692 | US10711020, Example 692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332437 (4-(1-(2-chloro- 6-(trifluoro- methyl)benzoyl)- 6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332482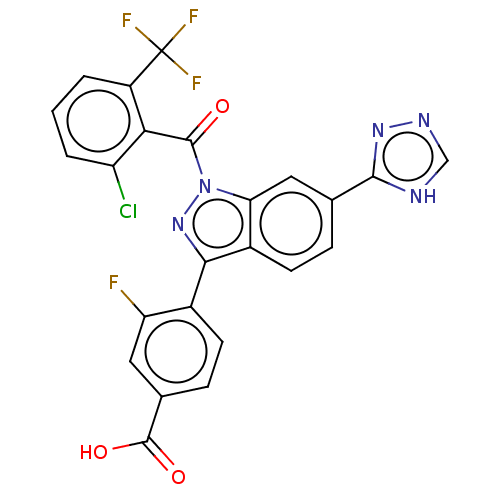 (4-(1-(2-chloro-6-(trifluoromethyl)benzoyl)-6-(4H-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382638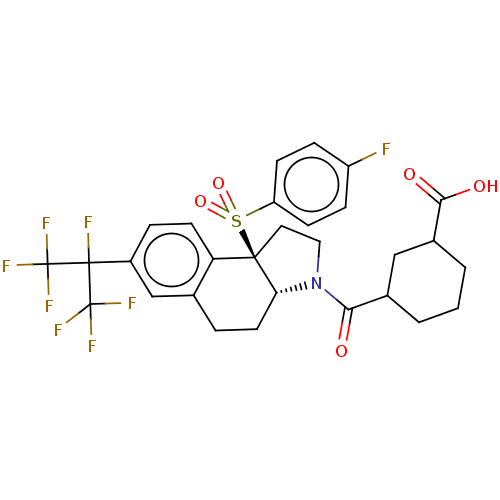 (US10273259, Example 347 | US10273259, Example 348 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332518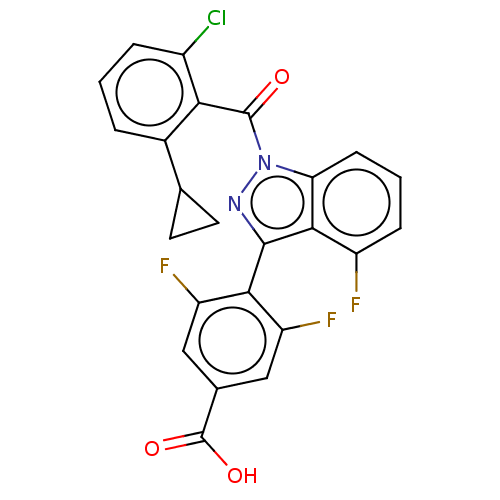 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-4- fluoro-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332517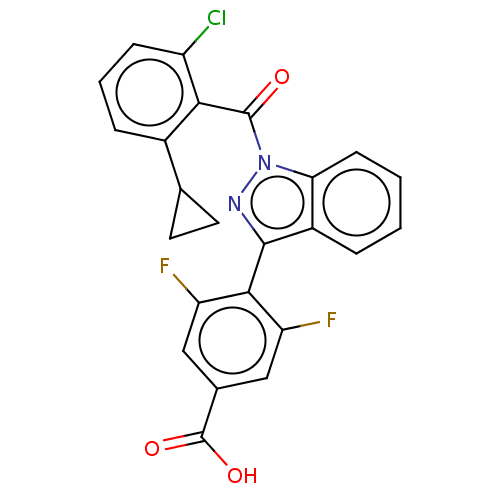 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-1H- indazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332511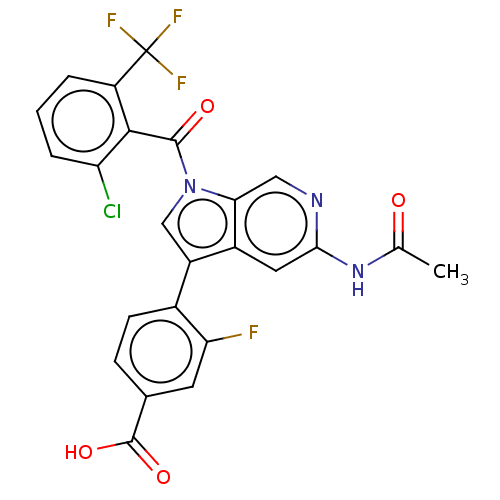 (4-(5-acetamido-1-(2-chloro-6-(trifluoromethyl)-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332411 (4-(1-(2-chloro-6-cyclopropylbenzoyl)-1H-pyrazolo[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332434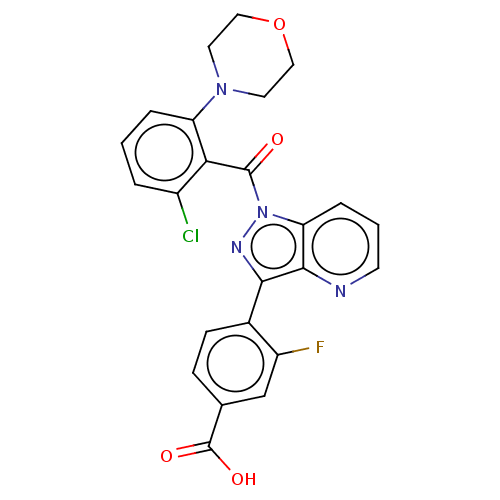 (4-(1-(2- chloro-6- morpholino- benzoyl)-1H- pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332516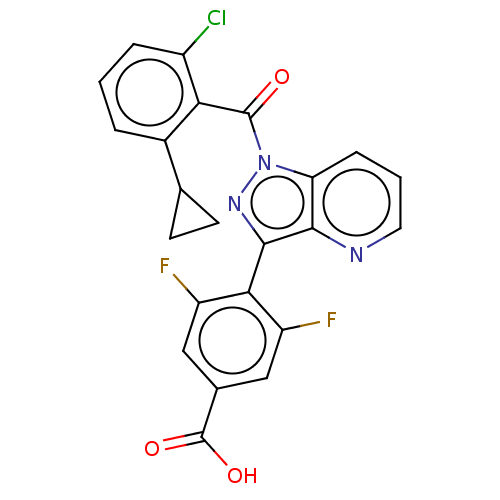 (4-(1-(2-chloro-6-cyclopropylbenzoyl)-1H-pyrazolo[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM383117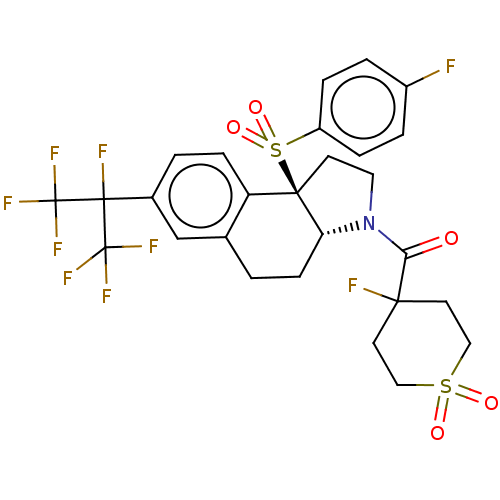 ((4-fluoro-1,1-dioxidotetrahydro-2H-thiopyran-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332519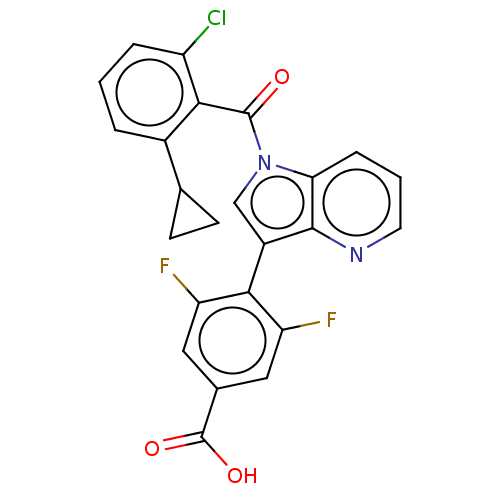 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-1H- pyrrolo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382482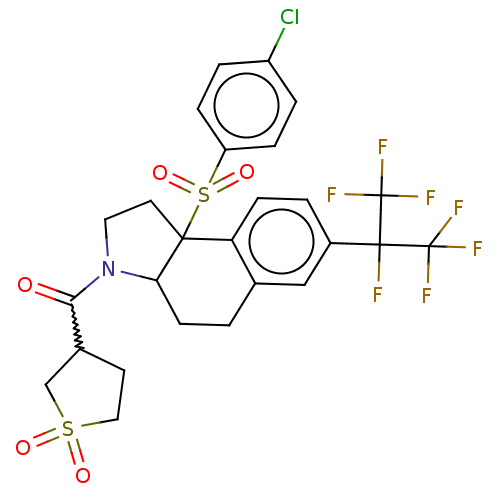 (US10273259, Example 191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM50446283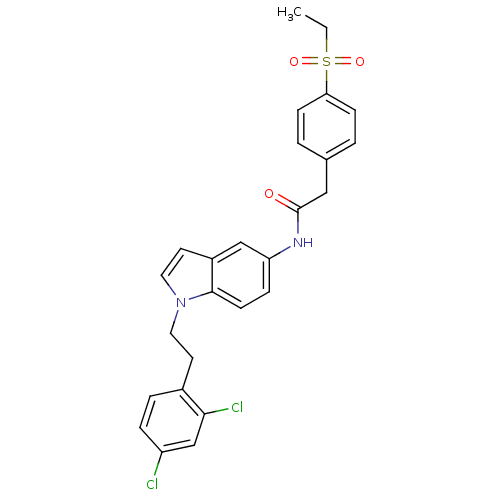 (CHEMBL3109235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inverse agonist activity at RORgammat in mouse Th17 cells assessed as effect on cell differentiation measured as IL-17 level after 3 days by ELISA | ACS Med Chem Lett 5: 65-8 (2014) Article DOI: 10.1021/ml4003875 BindingDB Entry DOI: 10.7270/Q2794654 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM50445880 (CHEMBL3105670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RORgammat receptor ligand binding domain in mouse spleen CD4+ T cells assessed as inhibition of IL-17 production after 3 days by ELISA | Bioorg Med Chem 22: 692-702 (2014) Article DOI: 10.1016/j.bmc.2013.12.021 BindingDB Entry DOI: 10.7270/Q2Z039M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382950 (US10273259, Example 661 | US10273259, Example 663 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382635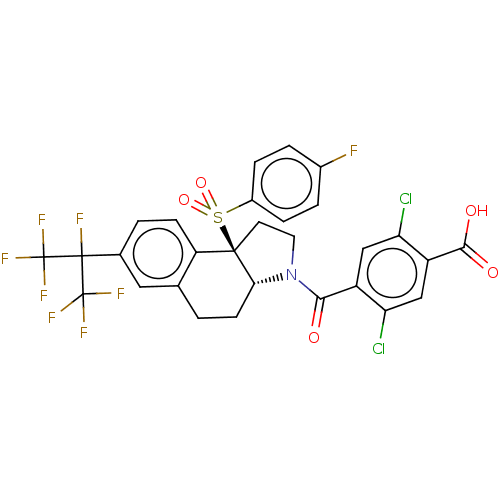 (US10273259, Example 344 | US10711020, Example 344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM50445883 (CHEMBL3105690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RORgammat receptor ligand binding domain in mouse spleen CD4+ T cells assessed as inhibition of IL-17 production after 3 days by ELISA | Bioorg Med Chem 22: 692-702 (2014) Article DOI: 10.1016/j.bmc.2013.12.021 BindingDB Entry DOI: 10.7270/Q2Z039M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332521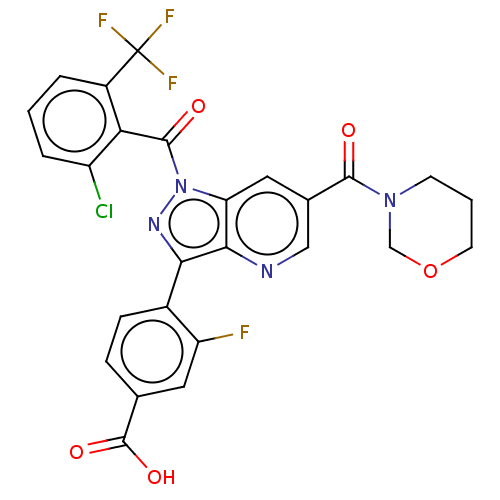 (4-(1-(2-chloro-6-(trifluoromethyl)benzoyl)-6-(1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382866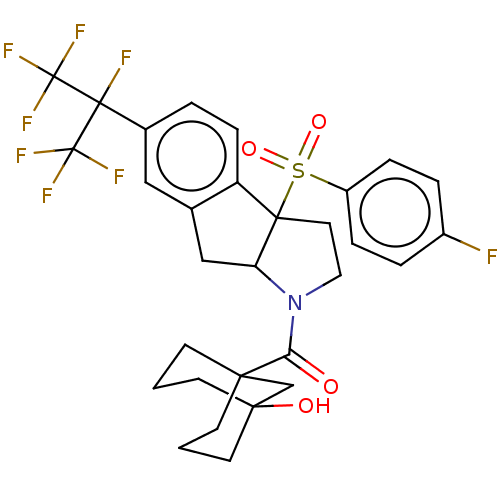 (US10273259, Example 577 | US10711020, Example 577) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382439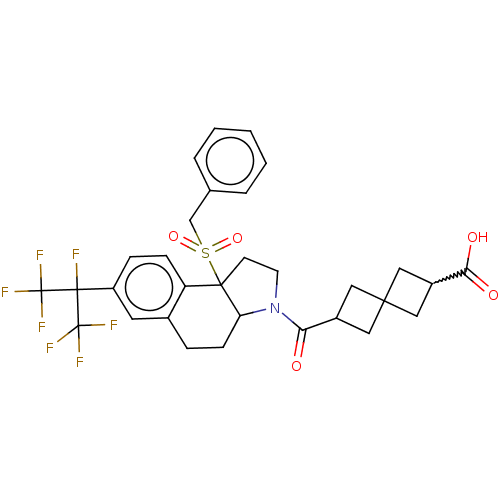 (US10273259, Example 150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332534 (4-(1-(2-chloro-6-cyclopentylbenzoyl)-1H-pyrazolo[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332477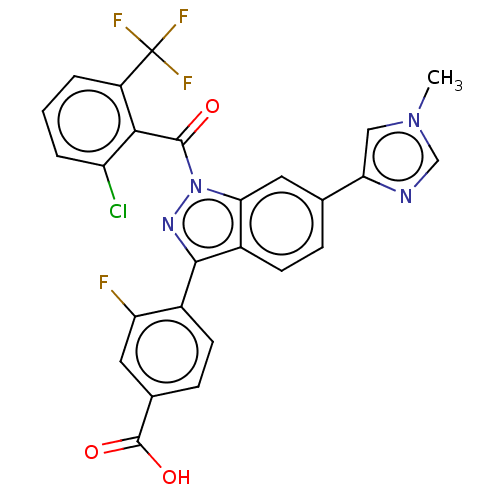 (4-(1-(2-chloro-6-(trifluoromethyl)benzoyl)-6-(1-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382696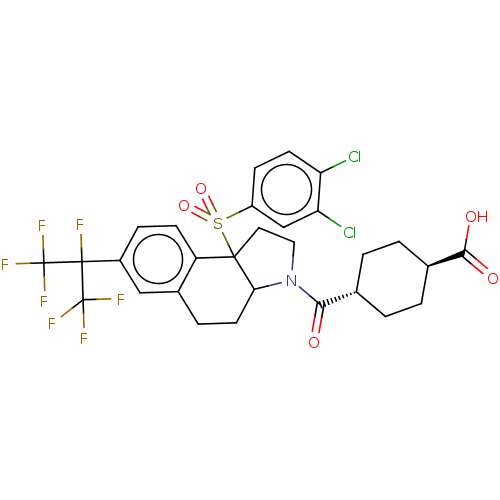 (US10273259, Example 405 | US10711020, Example 405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382654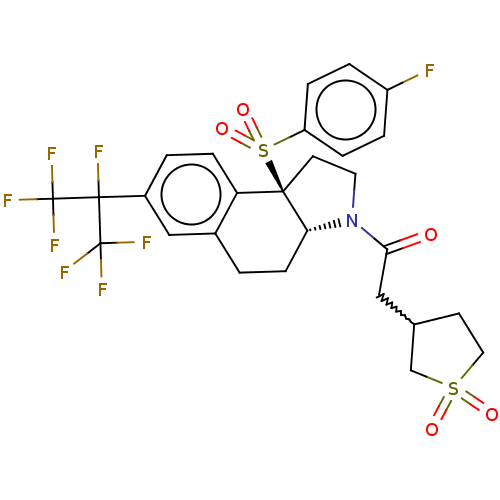 (US10273259, Example 363 | US10711020, Example 363) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382549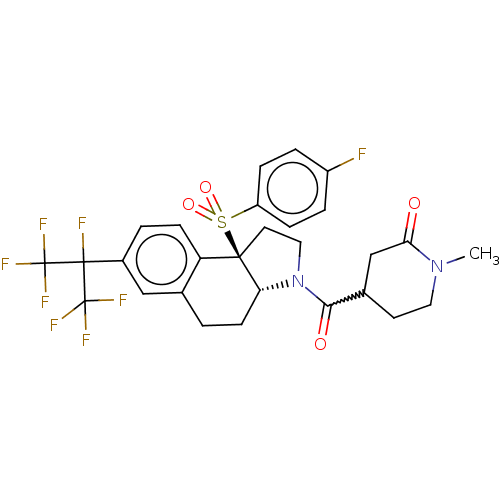 (US10273259, Example 258 | US10711020, Example 258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM50445879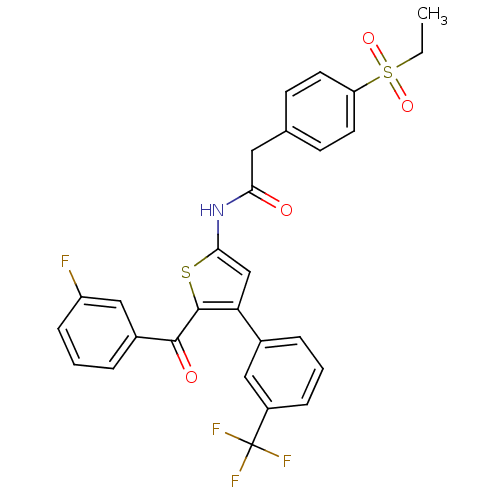 (CHEMBL3105672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RORgammat receptor ligand binding domain in mouse spleen CD4+ T cells assessed as inhibition of IL-17 production after 3 days by ELISA | Bioorg Med Chem 22: 692-702 (2014) Article DOI: 10.1016/j.bmc.2013.12.021 BindingDB Entry DOI: 10.7270/Q2Z039M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332510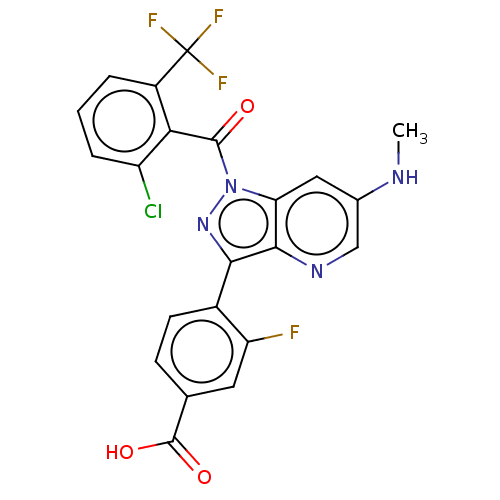 (4-(1-(2-chloro-6-(trifluoromethyl)benzoyl)-6-(meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382681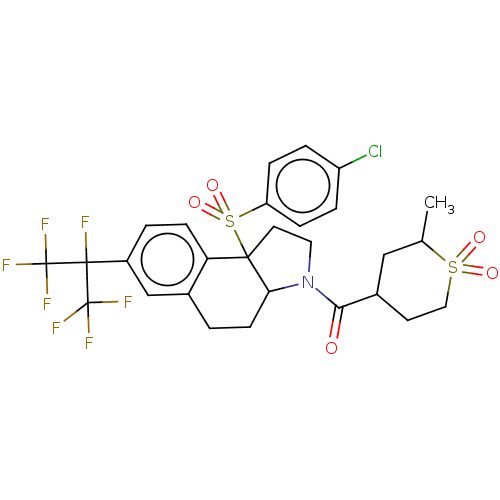 (US10273259, Example 390 | US10273259, Example 391 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382531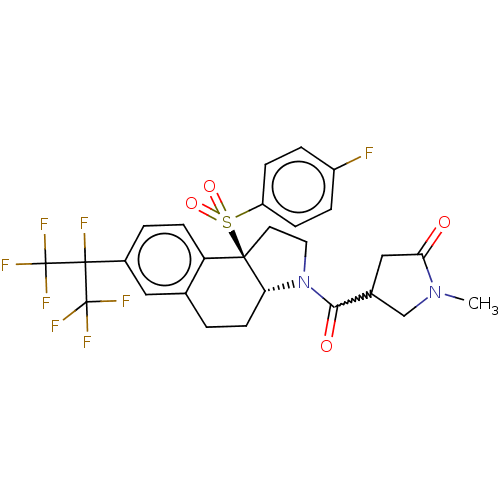 (US10273259, Example 240 | US10711020, Example 240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM383125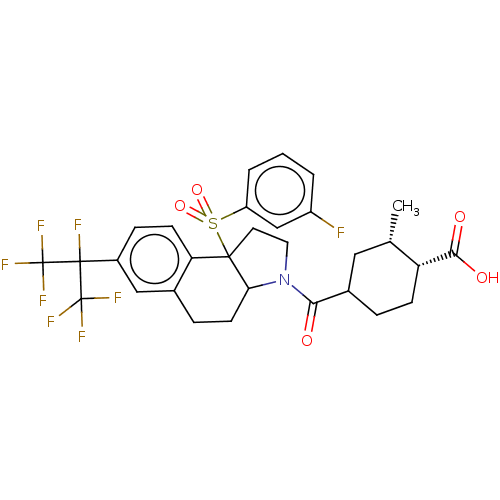 ((1R,2S)-4-(9b-((3-fluorophenyl)sulfonyl)-7-(perflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM336581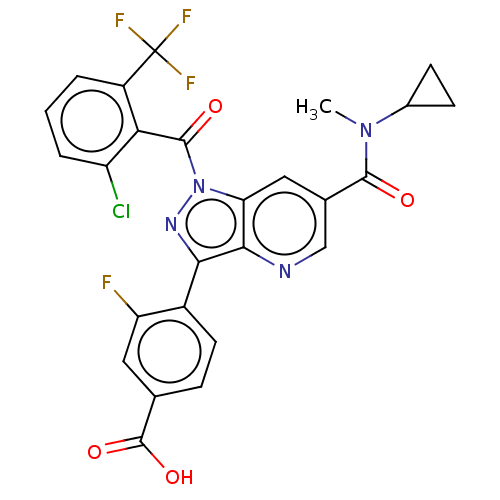 (4-(1-(2-chloro-6- (trifluoromethyl) benzoyl)-6- (c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332470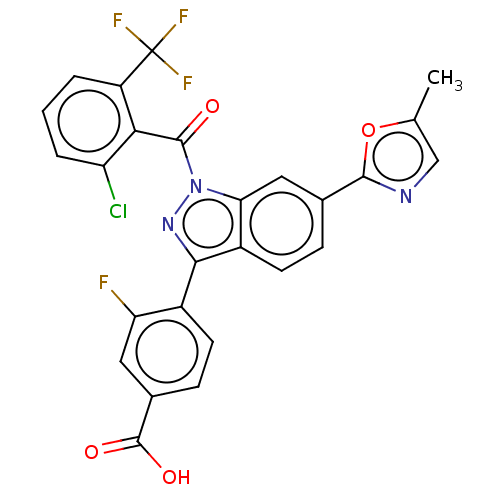 (4-(1-(2-chloro- 6- (trifluoromethyl) benzoyl)-6-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382738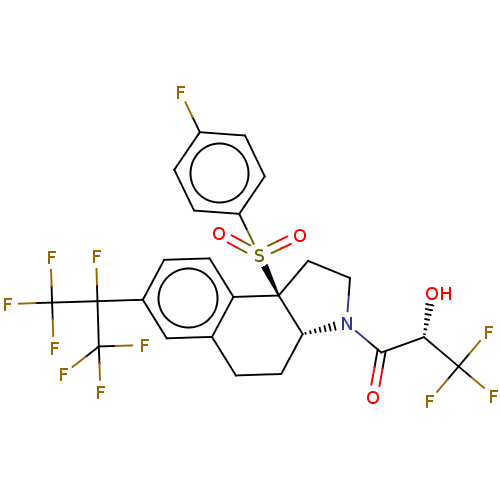 (US10273259, Example 447 | US10711020, Example 447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382706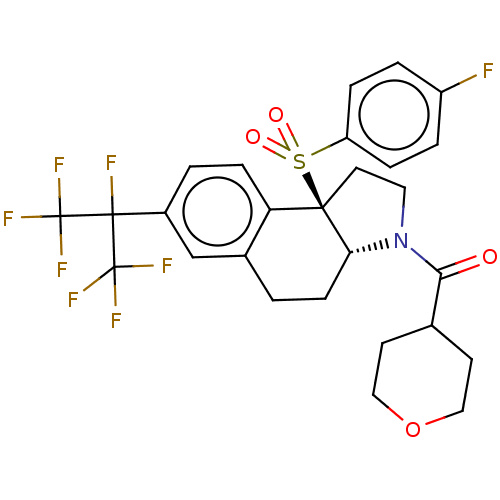 (US10273259, Example 415 | US10711020, Example 415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382530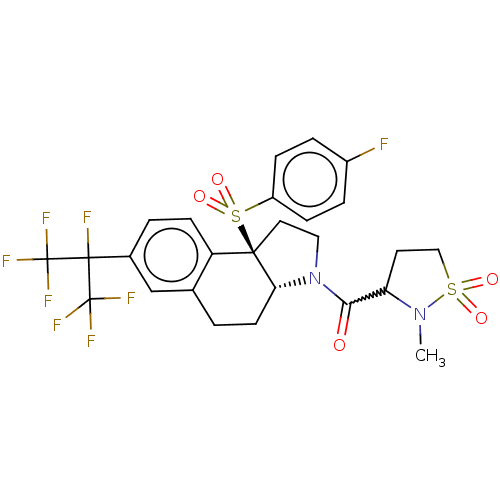 (US10273259, Example 239 | US10711020, Example 239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332436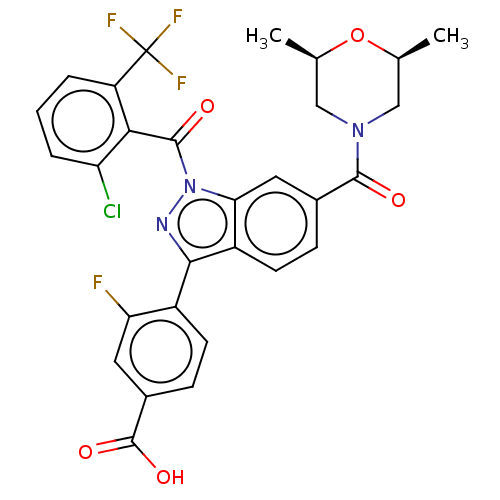 (4-(1-(2-chloro-6- (trifluoromethyl) benzoyl)-6- ((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM332484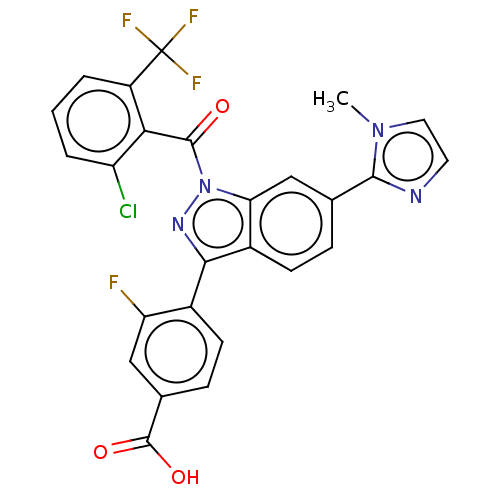 (4-(1-(2-chloro-6- (trifluoromethyl) benzoyl)-6-(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description To identify novel antagonists of RORgammaT, an assay was developed which employs the interaction of RORgammaT with its co-activator peptide SRC1_2. T... | US Patent US9745265 (2017) BindingDB Entry DOI: 10.7270/Q29G5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382997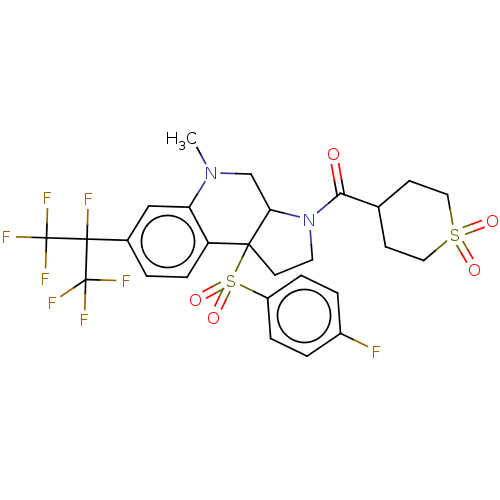 (US10273259, Example 708 | US10711020, Example 708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382805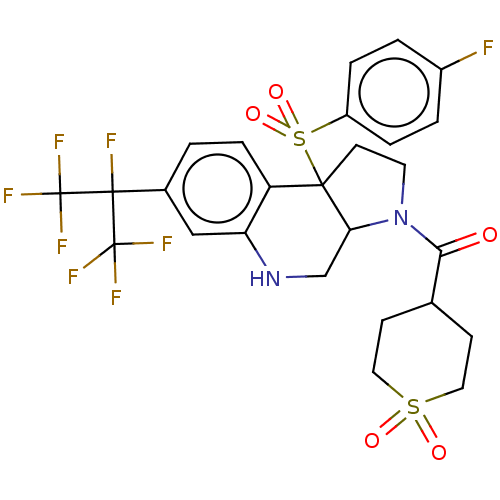 (US10273259, Example 516 | US10711020, Example 516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382954 (US10273259, Example 665 | US10711020, Example 665) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Mus musculus) | BDBM382704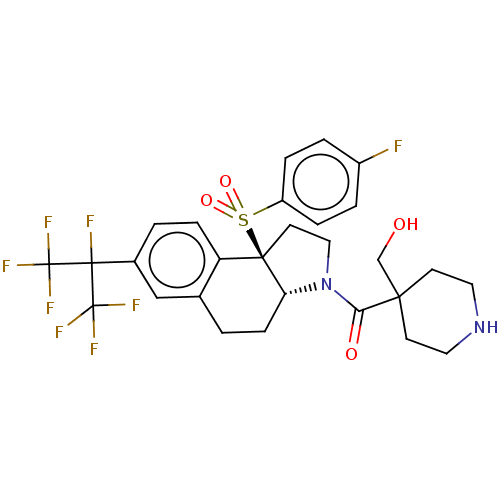 (US10273259, Example 413 | US10711020, Example 413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck | Assay Description The binding of potential ligands to RORγ is measured by competition with [3H]25-hydroxycholesterol (Perkin Elmer NET674250UC) using a scintillat... | Bioorg Med Chem Lett 18: 3456-61 (2008) BindingDB Entry DOI: 10.7270/Q2MW2KG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1130 total ) | Next | Last >> |