

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 2 (Mus musculus) | BDBM50506088 (CHEMBL4462143) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine Curated by ChEMBL | Assay Description Inhibition of CXCR2-mediated chemotaxis in mouse BAF3 cells | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111853 BindingDB Entry DOI: 10.7270/Q2057K6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Mus musculus) | BDBM50255479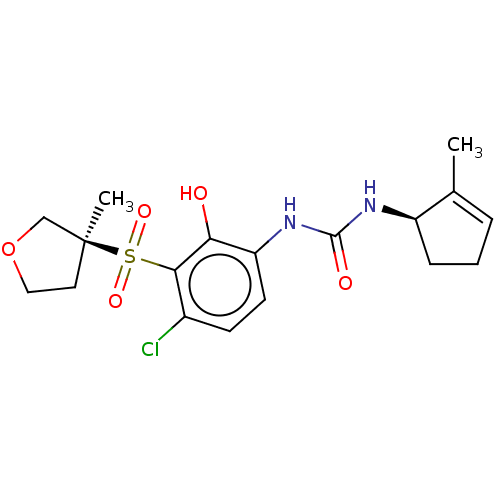 (CHEMBL4067429) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurosciences Therapeutic Area Unit , GSK Pharmaceuticals R&D , 898 Halei Road, Zhangjiang Hi-Tech Park , Pudong , Shanghai 201203 , P. R. China. Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 in C57 mouse whole blood assessed as inhibition of GROalpha-stimulated CD11b upregulation preincubated for 1 hr followed... | J Med Chem 61: 2518-2532 (2018) Article DOI: 10.1021/acs.jmedchem.7b01854 BindingDB Entry DOI: 10.7270/Q2959M1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Mus musculus) | BDBM50200880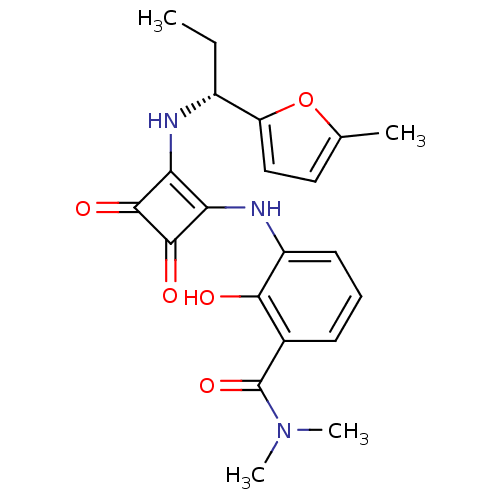 ((R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurosciences Therapeutic Area Unit , GSK Pharmaceuticals R&D , 898 Halei Road, Zhangjiang Hi-Tech Park , Pudong , Shanghai 201203 , P. R. China. Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 in C57 mouse whole blood assessed as inhibition of GROalpha-stimulated CD11b upregulation preincubated for 1 hr followed... | J Med Chem 61: 2518-2532 (2018) Article DOI: 10.1021/acs.jmedchem.7b01854 BindingDB Entry DOI: 10.7270/Q2959M1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||