

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Oryctolagus cuniculus (Rabbit)) | BDBM369458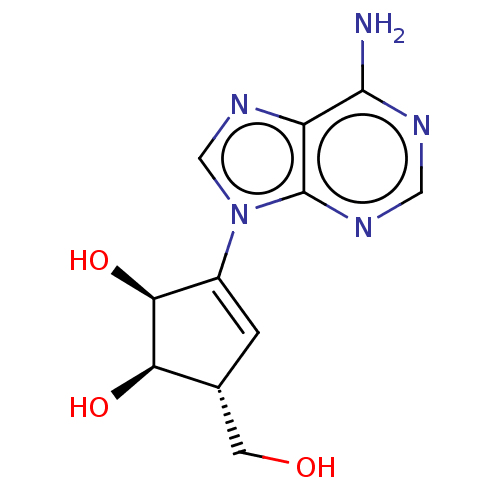 (US10227373, Compound D-Isoneplanocin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description As a consequence of "D"-Isoneplanocin and "L"-Isoneplanocin, being isomers of Neplanocin A, which on one hand is a potent inhibitor of S-adenosylhomo... | J Med Chem 51: 1904-12 (2008) BindingDB Entry DOI: 10.7270/Q24Q7X8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Oryctolagus cuniculus (Rabbit)) | BDBM50140074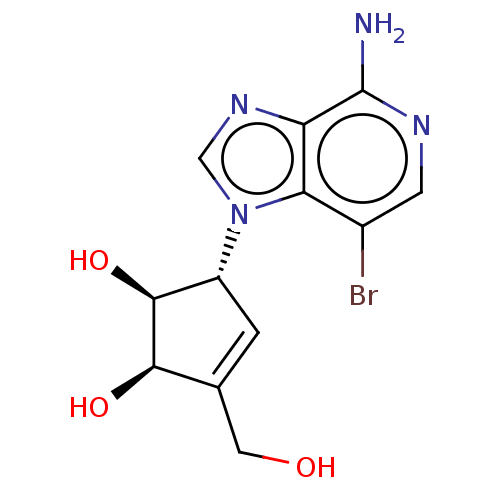 (CHEMBL2059155 | US10227373, Compound 3-Bromo-3-dea...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description As a consequence of "D"-Isoneplanocin and "L"-Isoneplanocin, being isomers of Neplanocin A, which on one hand is a potent inhibitor of S-adenosylhomo... | J Med Chem 51: 1904-12 (2008) BindingDB Entry DOI: 10.7270/Q24Q7X8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Oryctolagus cuniculus (Rabbit)) | BDBM50096906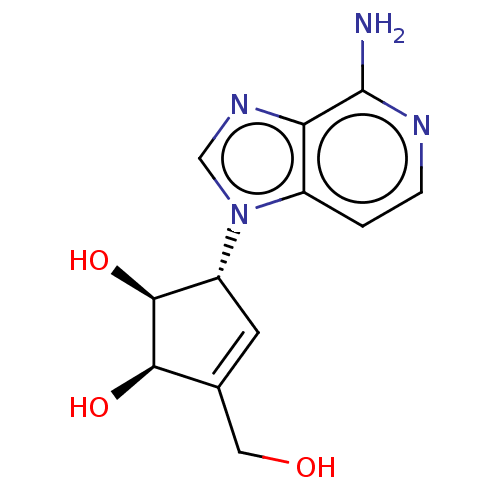 (CHEMBL154745 | US10227373, Compound D-3-Deazaisone...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description As a consequence of "D"-Isoneplanocin and "L"-Isoneplanocin, being isomers of Neplanocin A, which on one hand is a potent inhibitor of S-adenosylhomo... | J Med Chem 51: 1904-12 (2008) BindingDB Entry DOI: 10.7270/Q24Q7X8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Oryctolagus cuniculus (Rabbit)) | BDBM50465905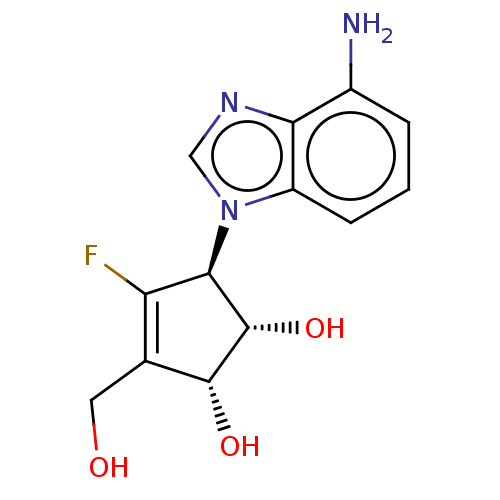 (CHEMBL4285617) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Auburn University Curated by ChEMBL | Assay Description Inhibition of SAHase from rabbit erythrocytes using S-adenosyl-L-homocysteine as substrate preincubated for 5 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3674-3675 (2018) Article DOI: 10.1016/j.bmcl.2018.10.030 BindingDB Entry DOI: 10.7270/Q2CN76KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Oryctolagus cuniculus (Rabbit)) | BDBM369457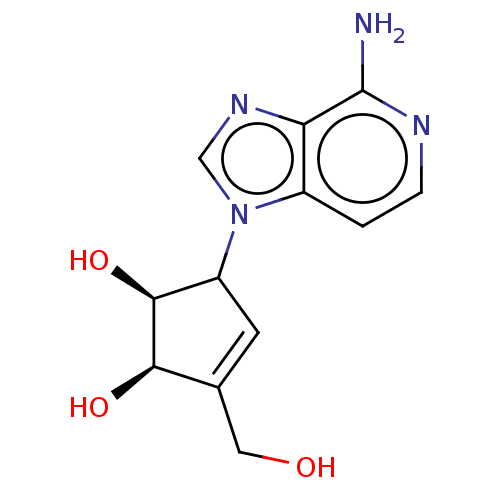 (US10227373, Compound 3-Deazaneplanocin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description As a consequence of "D"-Isoneplanocin and "L"-Isoneplanocin, being isomers of Neplanocin A, which on one hand is a potent inhibitor of S-adenosylhomo... | J Med Chem 51: 1904-12 (2008) BindingDB Entry DOI: 10.7270/Q24Q7X8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Oryctolagus cuniculus (Rabbit)) | BDBM369459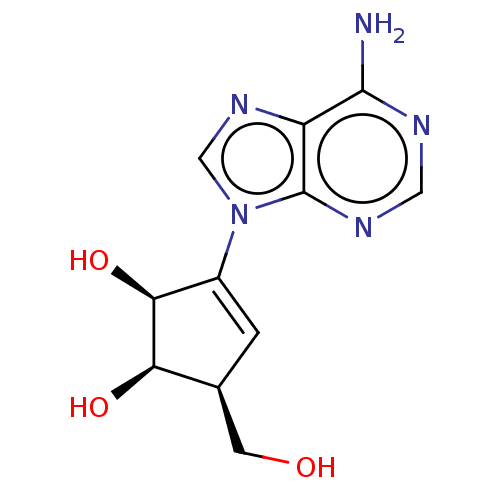 (US10227373, Compound L-Isoneplanocin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description As a consequence of "D"-Isoneplanocin and "L"-Isoneplanocin, being isomers of Neplanocin A, which on one hand is a potent inhibitor of S-adenosylhomo... | J Med Chem 51: 1904-12 (2008) BindingDB Entry DOI: 10.7270/Q24Q7X8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Oryctolagus cuniculus (Rabbit)) | BDBM369461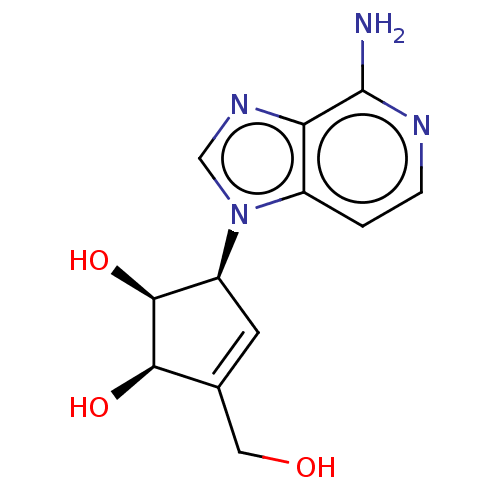 (US10227373, Compound L-3-Deazaisoneplanocin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description As a consequence of "D"-Isoneplanocin and "L"-Isoneplanocin, being isomers of Neplanocin A, which on one hand is a potent inhibitor of S-adenosylhomo... | J Med Chem 51: 1904-12 (2008) BindingDB Entry DOI: 10.7270/Q24Q7X8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||