 Found 10 hits of ec50 for UniProtKB: P20813
Found 10 hits of ec50 for UniProtKB: P20813 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50088447
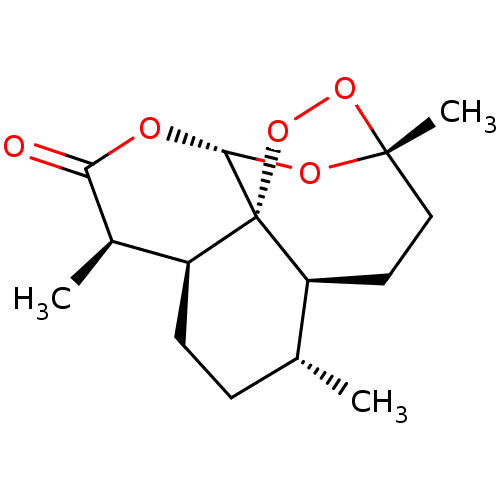
((+)-Artemisinin | CHEBI:223316)Show SMILES [H][C@@]12CC[C@@H](C)[C@]3([H])CC[C@@]4(C)OO[C@@]13[C@]([H])(OC(=O)[C@@H]2C)O4 |r| Show InChI InChI=1S/C15H22O5/c1-8-4-5-11-9(2)12(16)17-13-15(11)10(8)6-7-14(3,18-13)19-20-15/h8-11,13H,4-7H2,1-3H3/t8-,9-,10+,11+,13-,14-,15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Induction of CYP2B6 in human hepatocytes after 72 hrs relative to vehicle-treated control |
Drug Metab Dispos 40: 1757-64 (2012)
Article DOI: 10.1124/dmd.112.045765
BindingDB Entry DOI: 10.7270/Q2QJ7K0B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50370232

(BA-41166E | L-5103 | RIFAMPIN | Rifadin | Rifampic...)Show SMILES CO[C@H]1\C=C\O[C@@]2(C)Oc3c(C2=O)c2c(O)c(\C=N\N4CCN(C)CC4)c(NC(=O)\C(C)=C/C=C/[C@H](C)[C@H](O)[C@@H](C)[C@@H](O)[C@@H](C)[C@H](OC(C)=O)[C@@H]1C)c(O)c2c(O)c3C |r,c:33,t:3,35| Show InChI InChI=1S/C43H58N4O12/c1-21-12-11-13-22(2)42(55)45-33-28(20-44-47-17-15-46(9)16-18-47)37(52)30-31(38(33)53)36(51)26(6)40-32(30)41(54)43(8,59-40)57-19-14-29(56-10)23(3)39(58-27(7)48)25(5)35(50)24(4)34(21)49/h11-14,19-21,23-25,29,34-35,39,49-53H,15-18H2,1-10H3,(H,45,55)/b12-11+,19-14+,22-13-,44-20+/t21-,23+,24+,25+,29-,34-,35+,39+,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Amgen
Curated by ChEMBL
| Assay Description
Induction of CYP2B6 activity in human donor hepatocytes assessed as hydroxybupropion formation after 48 hrs by LC/MS method |
Drug Metab Dispos 41: 270-4 (2013)
Article DOI: 10.1124/dmd.112.047118
BindingDB Entry DOI: 10.7270/Q2MK6GWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50577263

(CHEMBL4850236)Show SMILES [H][C@@]12C[C@H](C[C@]1([H])CN(CC1CCOCC1)C2)Nc1ccc(nn1)-c1cc(F)cc(F)c1F |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of CYP2B6 in human hepatocytes |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00363
BindingDB Entry DOI: 10.7270/Q22B92V4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM17292

((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3CC[C@@]21[H] Show InChI InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
Amgen
Curated by ChEMBL
| Assay Description
Induction of CYP2B6 activity in human donor hepatocytes assessed as hydroxybupropion formation after 48 hrs by LC/MS method |
Drug Metab Dispos 41: 270-4 (2013)
Article DOI: 10.1124/dmd.112.047118
BindingDB Entry DOI: 10.7270/Q2MK6GWS |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM372346
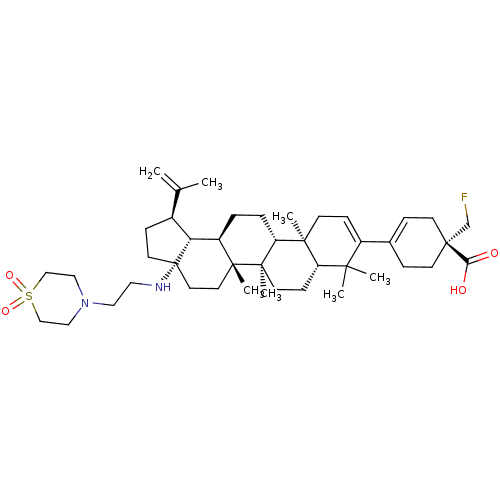
(Method A: (R)-4-((1R,3aS,5aR,5bR,7aR,11aS,11bR,13a...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC=C(C6=CC[C@](CF)(CC6)C(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)NCCN1CCS(=O)(=O)CC1 |r,t:18,20| Show InChI InChI=1S/C43H67FN2O4S/c1-29(2)31-12-19-43(45-22-23-46-24-26-51(49,50)27-25-46)21-20-40(6)33(36(31)43)8-9-35-39(5)15-13-32(38(3,4)34(39)14-16-41(35,40)7)30-10-17-42(28-44,18-11-30)37(47)48/h10,13,31,33-36,45H,1,8-9,11-12,14-28H2,2-7H3,(H,47,48)/t31-,33+,34-,35+,36+,39-,40+,41+,42+,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00879
BindingDB Entry DOI: 10.7270/Q26W9G2J |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50575174
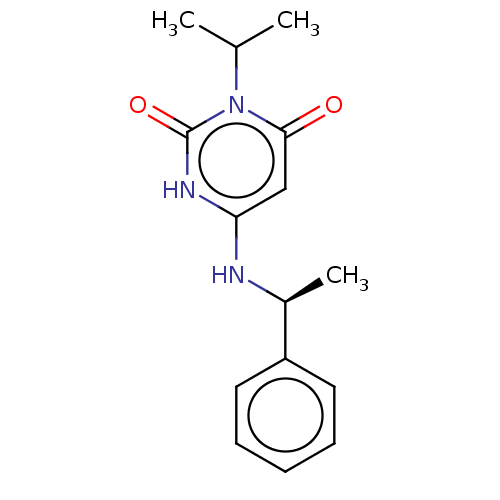
(Mavacamten) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Induction of CYP2B6 in human hepatocytes assessed as mRNA expression incubated for 3 days by RT-RTPCR analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01290
BindingDB Entry DOI: 10.7270/Q2SF30ZX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50249403
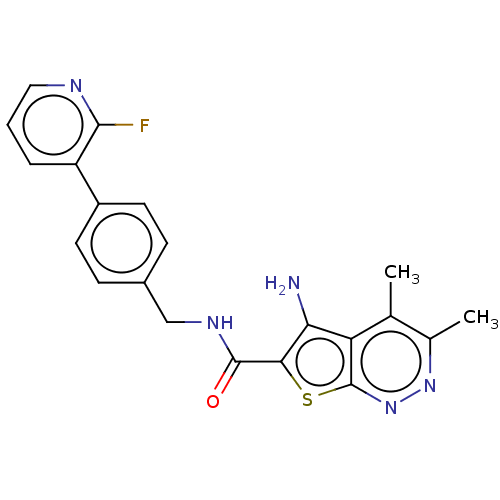
(CHEMBL4091821)Show SMILES Cc1nnc2sc(C(=O)NCc3ccc(cc3)-c3cccnc3F)c(N)c2c1C Show InChI InChI=1S/C21H18FN5OS/c1-11-12(2)26-27-21-16(11)17(23)18(29-21)20(28)25-10-13-5-7-14(8-6-13)15-4-3-9-24-19(15)22/h3-9H,10,23H2,1-2H3,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center
Curated by ChEMBL
| Assay Description
Induction of CYP2B6 in cryopreserved human hepatocytes measured after 48 hrs |
Bioorg Med Chem Lett 27: 2296-2301 (2017)
Article DOI: 10.1016/j.bmcl.2017.04.043
BindingDB Entry DOI: 10.7270/Q23B62J8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50451549
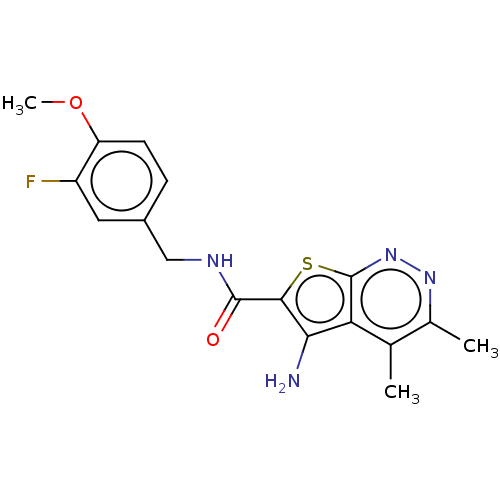
(CHEMBL4207737)Show InChI InChI=1S/C17H17FN4O2S/c1-8-9(2)21-22-17-13(8)14(19)15(25-17)16(23)20-7-10-4-5-12(24-3)11(18)6-10/h4-6H,7,19H2,1-3H3,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50437753
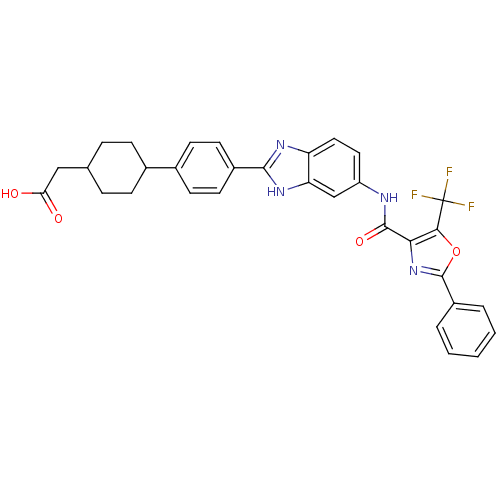
(CHEMBL2409564)Show SMILES OC(=O)CC1CCC(CC1)c1ccc(cc1)-c1nc2ccc(NC(=O)c3nc(oc3C(F)(F)F)-c3ccccc3)cc2[nH]1 |(55.73,-27.91,;54.2,-27.92,;53.41,-26.59,;53.44,-29.26,;51.9,-29.27,;51.14,-30.61,;49.6,-30.63,;48.82,-29.3,;49.57,-27.96,;51.11,-27.95,;47.28,-29.32,;46.53,-30.67,;44.99,-30.68,;44.2,-29.35,;44.95,-28.02,;46.49,-27.99,;42.67,-29.37,;41.78,-30.63,;40.31,-30.17,;38.97,-30.95,;37.64,-30.19,;37.64,-28.65,;36.3,-27.89,;34.97,-28.66,;34.98,-30.2,;33.63,-27.9,;32.24,-28.53,;31.2,-27.4,;31.96,-26.06,;33.47,-26.37,;34.61,-25.34,;36.08,-25.81,;34.29,-23.83,;35.93,-24.55,;29.67,-27.56,;28.76,-26.32,;27.23,-26.49,;26.61,-27.9,;27.53,-29.15,;29.06,-28.97,;38.96,-27.87,;40.29,-28.63,;41.76,-28.14,)| Show InChI InChI=1S/C32H27F3N4O4/c33-32(34,35)28-27(39-31(43-28)22-4-2-1-3-5-22)30(42)36-23-14-15-24-25(17-23)38-29(37-24)21-12-10-20(11-13-21)19-8-6-18(7-9-19)16-26(40)41/h1-5,10-15,17-19H,6-9,16H2,(H,36,42)(H,37,38)(H,40,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology
Curated by ChEMBL
| Assay Description
Induction of CYP2B6 (unknown origin) |
Bioorg Med Chem Lett 23: 4713-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.081
BindingDB Entry DOI: 10.7270/Q21J9C63 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50003659
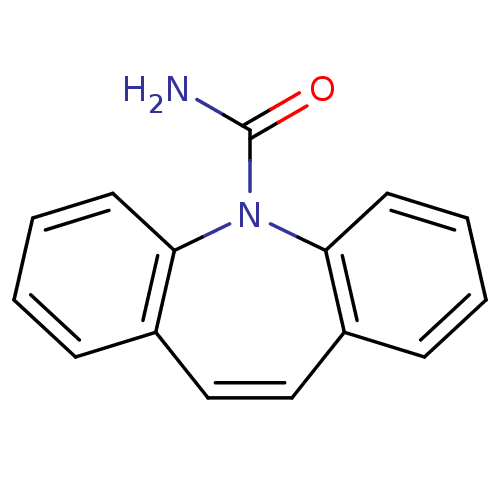
(5H-dibenzo[b,f]azepine-5-carboxamide | CARBAMAZEPI...)Show InChI InChI=1S/C15H12N2O/c16-15(18)17-13-7-3-1-5-11(13)9-10-12-6-2-4-8-14(12)17/h1-10H,(H2,16,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a |
Amgen
Curated by ChEMBL
| Assay Description
Induction of CYP2B6 activity in human donor hepatocytes assessed as hydroxybupropion formation after 48 hrs by LC/MS method |
Drug Metab Dispos 41: 270-4 (2013)
Article DOI: 10.1124/dmd.112.047118
BindingDB Entry DOI: 10.7270/Q2MK6GWS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data