 Found 25 hits of ki for UniProtKB: P20813
Found 25 hits of ki for UniProtKB: P20813 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM85509
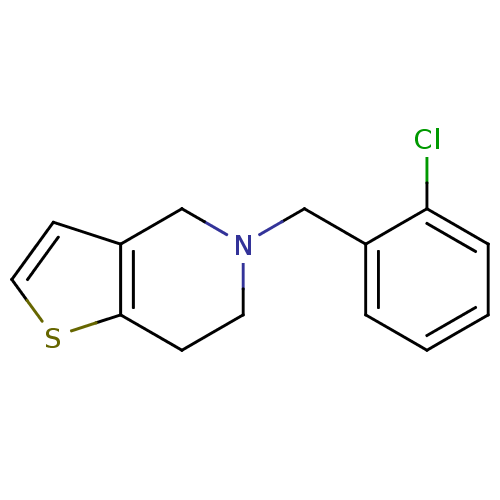
(CAS_55142-85-3 | NSC_5472 | Ticlopidine)Show InChI InChI=1S/C14H14ClNS/c15-13-4-2-1-3-11(13)9-16-7-5-14-12(10-16)6-8-17-14/h1-4,6,8H,5,7,9-10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 measured by bupropion hydroxylation using human liver microsomes |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50333117
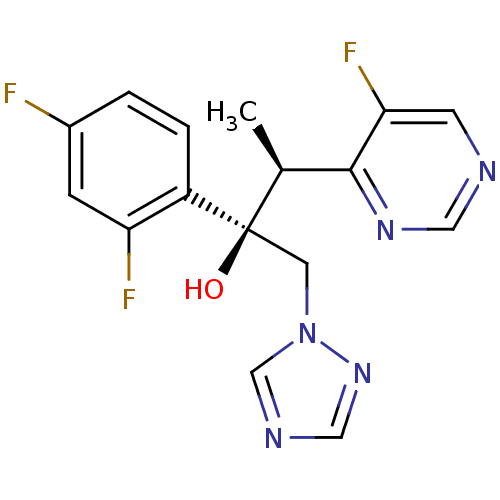
((2R,3S)-2,3-bis(2,4-difluorophenyl)-1-(1H-1,2,4-tr...)Show SMILES C[C@@H](c1ncncc1F)[C@](O)(Cn1cncn1)c1ccc(F)cc1F |r| Show InChI InChI=1S/C16H14F3N5O/c1-10(15-14(19)5-20-7-22-15)16(25,6-24-9-21-8-23-24)12-3-2-11(17)4-13(12)18/h2-5,7-10,25H,6H2,1H3/t10-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human liver microsomes assessed as bupropion 4-hydroxylation after 15 mins by Dixon plot analysis |
Antimicrob Agents Chemother 53: 541-51 (2009)
Article DOI: 10.1128/AAC.01123-08
BindingDB Entry DOI: 10.7270/Q2H13285 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50333117

((2R,3S)-2,3-bis(2,4-difluorophenyl)-1-(1H-1,2,4-tr...)Show SMILES C[C@@H](c1ncncc1F)[C@](O)(Cn1cncn1)c1ccc(F)cc1F |r| Show InChI InChI=1S/C16H14F3N5O/c1-10(15-14(19)5-20-7-22-15)16(25,6-24-9-21-8-23-24)12-3-2-11(17)4-13(12)18/h2-5,7-10,25H,6H2,1H3/t10-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human liver microsomes assessed as efavirenz 8-hydroxylation after 10 mins by Dixon plot analysis |
Antimicrob Agents Chemother 53: 541-51 (2009)
Article DOI: 10.1128/AAC.01123-08
BindingDB Entry DOI: 10.7270/Q2H13285 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50397662
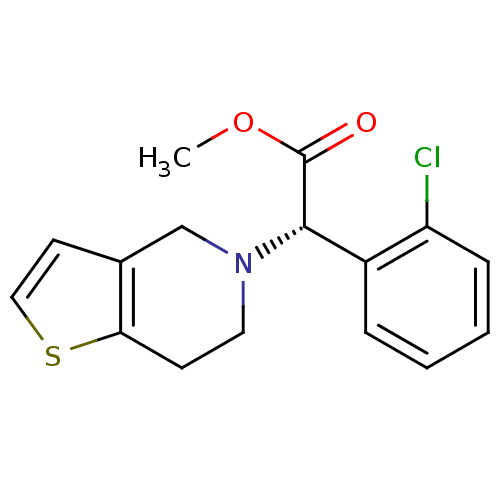
(CLOPIDOGREL)Show InChI InChI=1S/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 using human liver microsomes |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50397662

(CLOPIDOGREL)Show InChI InChI=1S/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP2B6 in human liver microsomes |
J Med Chem 55: 4896-933 (2012)
Article DOI: 10.1021/jm300065h
BindingDB Entry DOI: 10.7270/Q2PG1SVR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50318484

(2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...)Show SMILES CCCCCc1cc(O)c([C@@H]2C=C(C)CC[C@H]2C(C)=C)c(O)c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed type inhibition of human recombinant CYP2B6 expressed in baculovirus-infected insect cells using coumarin as substrate preincubated for 5 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50187243

(17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r| Show InChI InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 measured by 7-EFC O-deethylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM85509

(CAS_55142-85-3 | NSC_5472 | Ticlopidine)Show InChI InChI=1S/C14H14ClNS/c15-13-4-2-1-3-11(13)9-16-7-5-14-12(10-16)6-8-17-14/h1-4,6,8H,5,7,9-10H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 measured by bupropion hydroxylation using recombinant CYP2B6 |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50187243

(17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestra...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r| Show InChI InChI=1S/C20H24O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18,20)2/h1,5,7,12,16-18,21-22H,4,6,8-11H2,2H3/t16-,17-,18+,19+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 measured by 7-EFC O-deethylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM20607
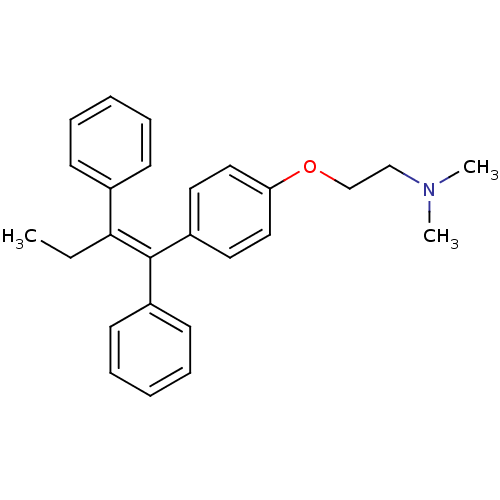
((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 measured by 7-EFC O-deethylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50397662

(CLOPIDOGREL)Show InChI InChI=1S/C16H16ClNO2S/c1-20-16(19)15(12-4-2-3-5-13(12)17)18-8-6-14-11(10-18)7-9-21-14/h2-5,7,9,15H,6,8,10H2,1H3/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 using recombinant CYP2B6 |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50418085
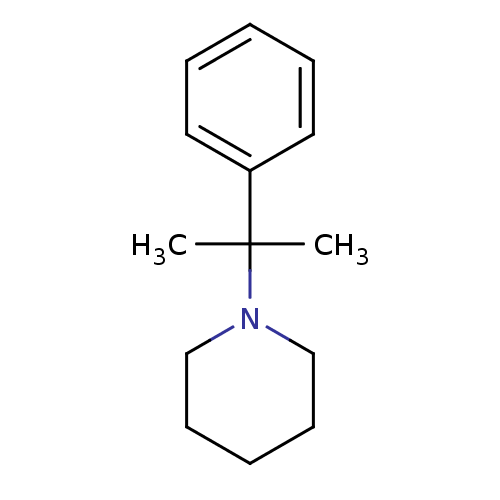
(CHEMBL1743344)Show InChI InChI=1S/C14H21N/c1-14(2,13-9-5-3-6-10-13)15-11-7-4-8-12-15/h3,5-6,9-10H,4,7-8,11-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50532215
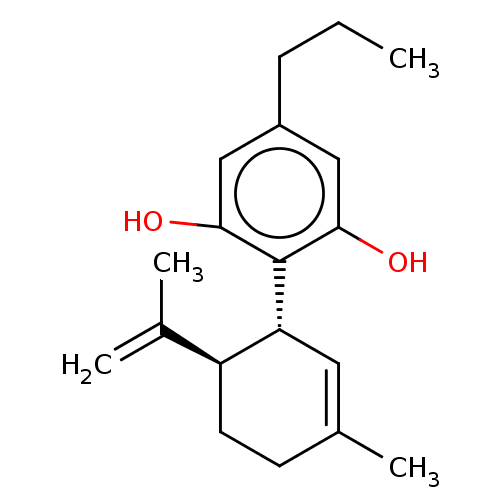
(CBD-V | CBDV | Cannabidivarin | GWP42006)Show SMILES [H][C@]1(CCC(C)=C[C@H]1c1c(O)cc(CCC)cc1O)C(C)=C |r,c:5| Show InChI InChI=1S/C19H26O2/c1-5-6-14-10-17(20)19(18(21)11-14)16-9-13(4)7-8-15(16)12(2)3/h9-11,15-16,20-21H,2,5-8H2,1,3-4H3/t15-,16+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50418086
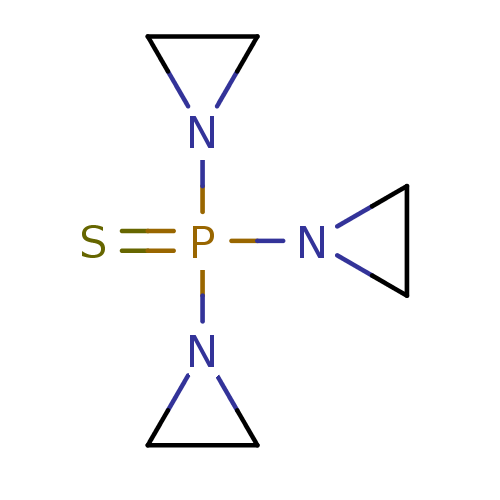
(THIOTEPA | Thioplex)Show InChI InChI=1S/C6H12N3PS/c11-10(7-1-2-7,8-3-4-8)9-5-6-9/h1-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
| DrugBank
PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 measured by bupropion hydroxylation using a recombinant system |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50061117

(6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol |...)Show InChI InChI=1S/C21H26O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-13,22H,5-8H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50418086
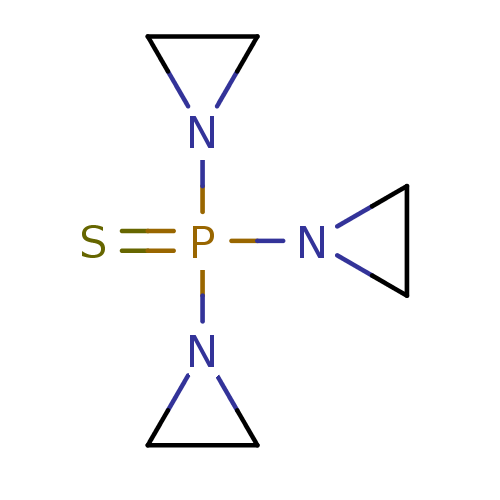
(THIOTEPA | Thioplex)Show InChI InChI=1S/C6H12N3PS/c11-10(7-1-2-7,8-3-4-8)9-5-6-9/h1-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
| DrugBank
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 measured by bupropion hydroxylation using human liver microsomes |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50088447
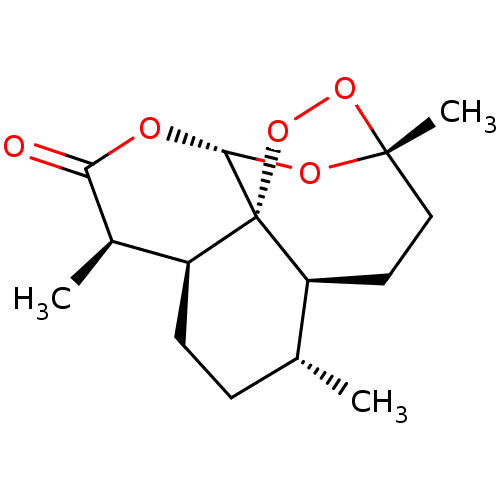
((+)-Artemisinin | CHEBI:223316)Show SMILES [H][C@@]12CC[C@@H](C)[C@]3([H])CC[C@@]4(C)OO[C@@]13[C@]([H])(OC(=O)[C@@H]2C)O4 |r| Show InChI InChI=1S/C15H22O5/c1-8-4-5-11-9(2)12(16)17-13-15(11)10(8)6-7-14(3,18-13)19-20-15/h8-11,13H,4-7H2,1-3H3/t8-,9-,10+,11+,13-,14-,15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of CYP2B6 in human liver microsomes incubated for 3 mins prior to NADPH addition measured after 10 mins by Dixon pl... |
Drug Metab Dispos 40: 1757-64 (2012)
Article DOI: 10.1124/dmd.112.045765
BindingDB Entry DOI: 10.7270/Q2QJ7K0B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50418087
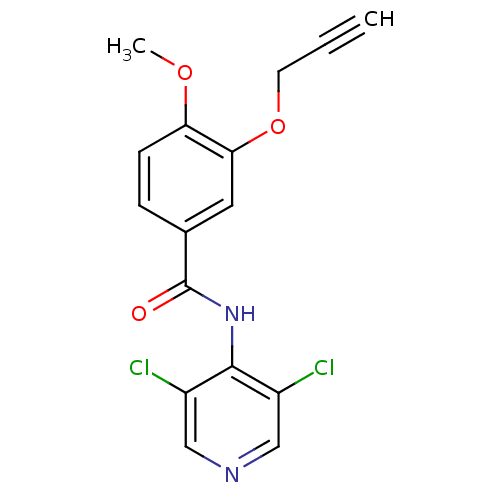
(CHEMBL1743351)Show InChI InChI=1S/C16H12Cl2N2O3/c1-3-6-23-14-7-10(4-5-13(14)22-2)16(21)20-15-11(17)8-19-9-12(15)18/h1,4-5,7-9H,6H2,2H3,(H,19,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 measured by N-(3,5-dichloro-4-pyridyl)-3- (cyclopentoxy)-4- methoxybenzamide (DCMB) hydroxyla... |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50310823

(CHEMBL1078442 | bergamottin)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6]\[#6](-[#6])=[#6]\[#6]-[#8]-c1c2ccoc2cc2oc(=O)ccc12 Show InChI InChI=1S/C21H22O4/c1-14(2)5-4-6-15(3)9-11-24-21-16-7-8-20(22)25-19(16)13-18-17(21)10-12-23-18/h5,7-10,12-13H,4,6,11H2,1-3H3/b15-9+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM82507
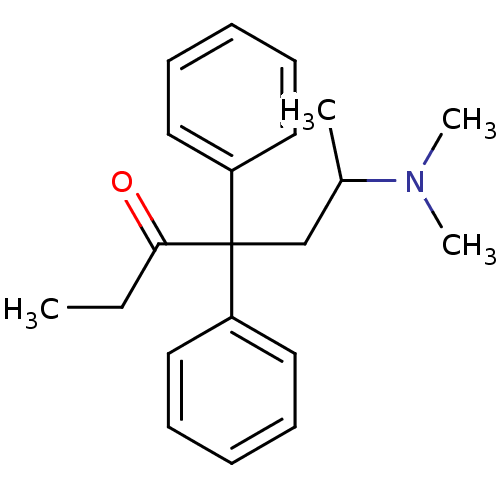
((+/-)-Methadone | CAS_5967-73-7 | METHADONE | Meth...)Show InChI InChI=1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan
Curated by ChEMBL
| Assay Description
Inactivation of C-terminal His4-tagged human CYP2B6 lacking 21 N-terminal residues expressed in Escherichia coli C41 (DE3) assessed as decreases in f... |
Drug Metab Dispos 40: 1765-70 (2012)
Article DOI: 10.1124/dmd.112.045971
BindingDB Entry DOI: 10.7270/Q21V5GP6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM83449
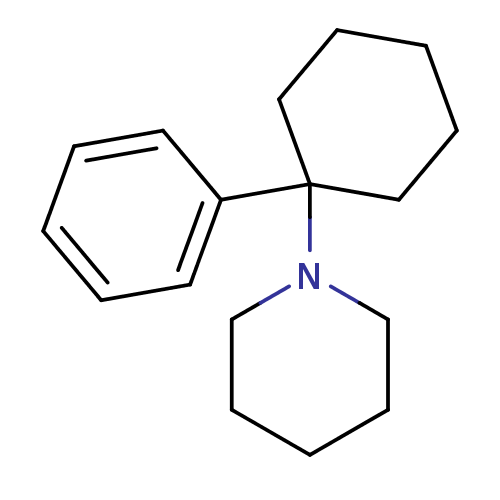
(1-(1-phenylcyclohexyl)piperidine;hydrochloride | M...)Show InChI InChI=1S/C17H25N/c1-4-10-16(11-5-1)17(12-6-2-7-13-17)18-14-8-3-9-15-18/h1,4-5,10-11H,2-3,6-9,12-15H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50088490

(CHEMBL3526979)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(=O)O[C@@H]3O[C@@H]([C@@H](O)[C@H](O)[C@H]3O)C(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 |r| Show InChI InChI=1S/C34H35Cl2FN2O12S/c1-34(2,3)38-31(44)17-6-8-23(22(12-17)39-52(47,48)25-9-7-16(10-20(25)36)15-4-5-15)49-24-14-21(37)18(11-19(24)35)13-26(40)50-33-29(43)27(41)28(42)30(51-33)32(45)46/h6-12,14-15,27-30,33,39,41-43H,4-5,13H2,1-3H3,(H,38,44)(H,45,46)/t27-,28-,29+,30-,33+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50363928
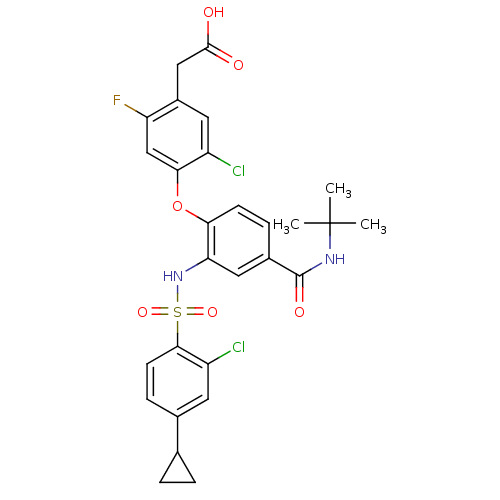
(CHEMBL1951575)Show SMILES CC(C)(C)NC(=O)c1ccc(Oc2cc(F)c(CC(O)=O)cc2Cl)c(NS(=O)(=O)c2ccc(cc2Cl)C2CC2)c1 Show InChI InChI=1S/C28H27Cl2FN2O6S/c1-28(2,3)32-27(36)17-6-8-23(39-24-14-21(31)18(11-19(24)29)13-26(34)35)22(12-17)33-40(37,38)25-9-7-16(10-20(25)30)15-4-5-15/h6-12,14-15,33H,4-5,13H2,1-3H3,(H,32,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human liver microsomes using bupropion as substrate after 5 to 30 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 2239-49 (2012)
Article DOI: 10.1124/dmd.112.047928
BindingDB Entry DOI: 10.7270/Q2Z039WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50061117

(6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol |...)Show InChI InChI=1S/C21H26O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h9-13,22H,5-8H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c00965
BindingDB Entry DOI: 10.7270/Q2057KWP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM50418086
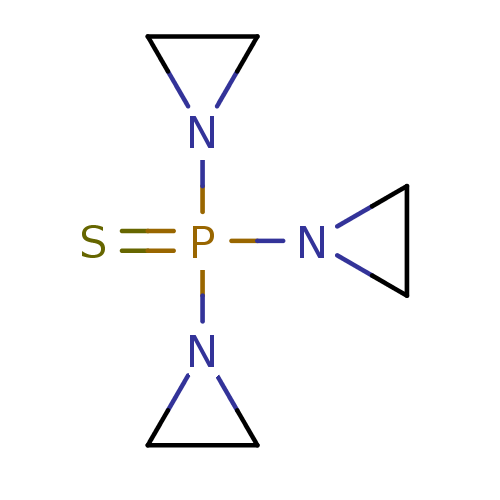
(THIOTEPA | Thioplex)Show InChI InChI=1S/C6H12N3PS/c11-10(7-1-2-7,8-3-4-8)9-5-6-9/h1-6H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
| DrugBank
PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2B6 |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data