 Found 5920 hits of ki for UniProtKB: P28223
Found 5920 hits of ki for UniProtKB: P28223 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50231459

(CHEMBL252818 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...)Show SMILES CC(=O)NC1CCc2ccc(CCN3CCN(CC3)c3nsc4ccccc34)cc12 |w:4.3| Show InChI InChI=1S/C24H28N4OS/c1-17(29)25-22-9-8-19-7-6-18(16-21(19)22)10-11-27-12-14-28(15-13-27)24-20-4-2-3-5-23(20)30-26-24/h2-7,16,22H,8-15H2,1H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775
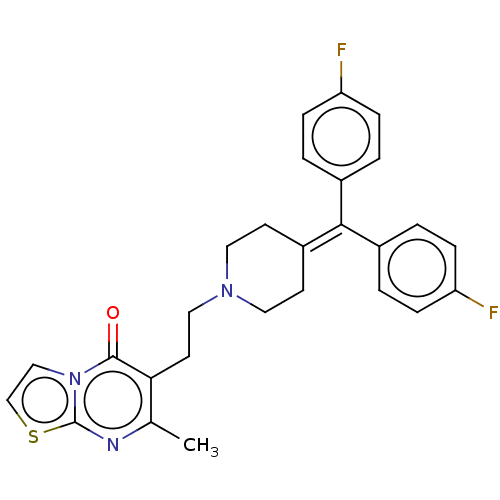
((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
Br J Pharmacol 115: 622-8 (1995)
Article DOI: 10.1111/j.1476-5381.1995.tb14977.x
BindingDB Entry DOI: 10.7270/Q2BR8QP0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50231448

(CHEMBL253022 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...)Show SMILES CCC(=O)NC1CCc2ccc(CCN3CCN(CC3)c3nsc4ccccc34)cc12 |w:5.4| Show InChI InChI=1S/C25H30N4OS/c1-2-24(30)26-22-10-9-19-8-7-18(17-21(19)22)11-12-28-13-15-29(16-14-28)25-20-5-3-4-6-23(20)31-27-25/h3-8,17,22H,2,9-16H2,1H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570555
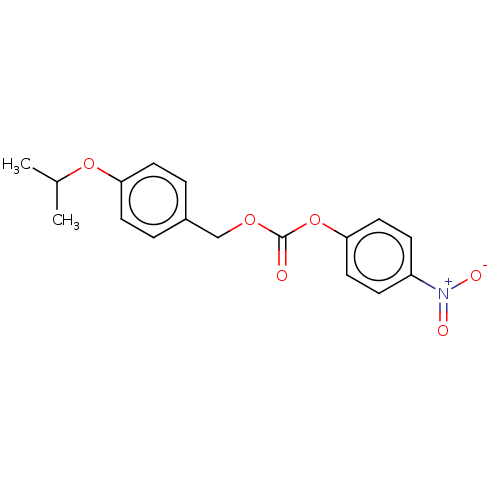
(US11440884, Example 18 | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)Oc2ccc(cc2)[N+]([O-])=O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50232153
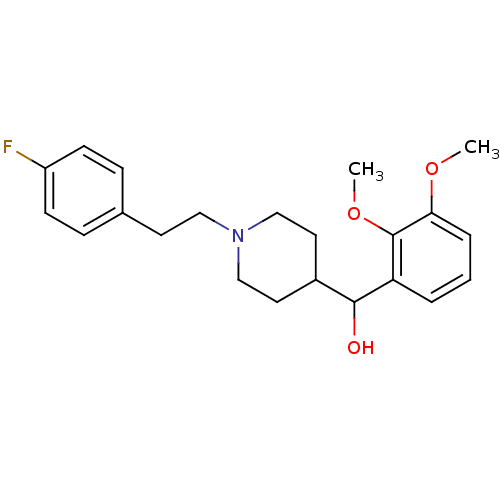
((1-(4-fluorophenethyl)piperidin-4-yl)(2,3-dimethox...)Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | -13.3 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570569
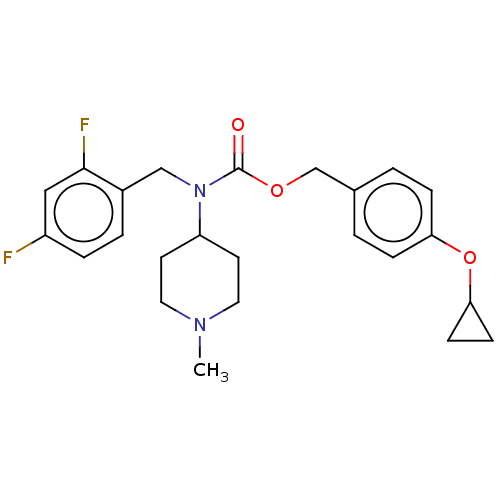
(US11440884, Example 30)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC2CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50231440

(CHEMBL253878 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...)Show SMILES O=C(Cc1ccccc1)NC1CCc2ccc(CCN3CCN(CC3)c3nsc4ccccc34)cc12 |w:10.10| Show InChI InChI=1S/C30H32N4OS/c35-29(21-22-6-2-1-3-7-22)31-27-13-12-24-11-10-23(20-26(24)27)14-15-33-16-18-34(19-17-33)30-25-8-4-5-9-28(25)36-32-30/h1-11,20,27H,12-19,21H2,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50068612
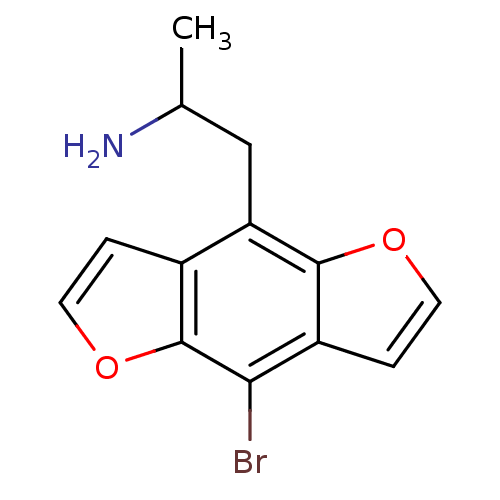
(2-(8-Bromo-benzo[1,2-b;4,5-b']difuran-4-yl)-1-meth...)Show InChI InChI=1S/C13H12BrNO2/c1-7(15)6-10-8-2-4-17-13(8)11(14)9-3-5-16-12(9)10/h2-5,7H,6,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding activity against cloned human 5-hydroxytryptamine 2A receptor using [125I]-DOI as the radioligand. |
J Med Chem 41: 5148-9 (1999)
Article DOI: 10.1021/jm9803525
BindingDB Entry DOI: 10.7270/Q2862FKX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM139371
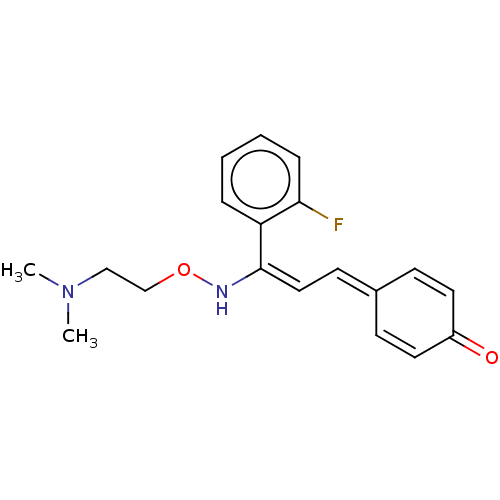
(eplivanserin)Show SMILES [#6]-[#7](-[#6])-[#6]-[#6]-[#8]-[#7]\[#6](=[#6]\[#6]=[#6]-1\[#6]=[#6]-[#6](=O)-[#6]=[#6]-1)-c1ccccc1F |c:11,15| Show InChI InChI=1S/C19H21FN2O2/c1-22(2)13-14-24-21-19(17-5-3-4-6-18(17)20)12-9-15-7-10-16(23)11-8-15/h3-12,21H,13-14H2,1-2H3/b19-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | -13.1 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50171676
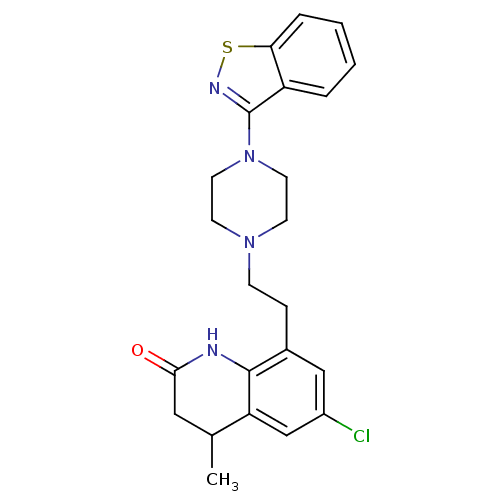
(8-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...)Show SMILES CC1CC(=O)Nc2c(CCN3CCN(CC3)c3nsc4ccccc34)cc(Cl)cc12 Show InChI InChI=1S/C23H25ClN4OS/c1-15-12-21(29)25-22-16(13-17(24)14-19(15)22)6-7-27-8-10-28(11-9-27)23-18-4-2-3-5-20(18)30-26-23/h2-5,13-15H,6-12H2,1H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity for 5-hydroxytryptamine 2A receptor expressed in 3T3 cells |
Bioorg Med Chem Lett 15: 4560-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.097
BindingDB Entry DOI: 10.7270/Q27S7NBQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099273
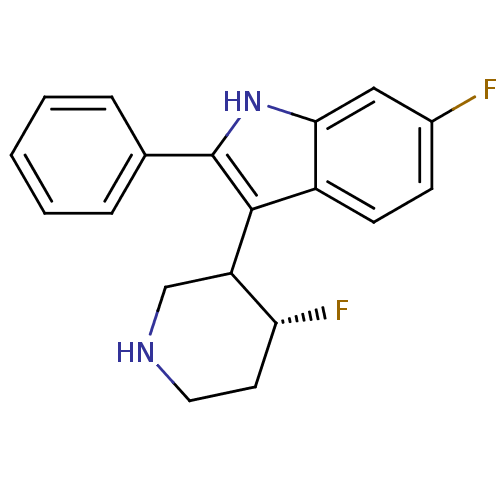
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099273

(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570568
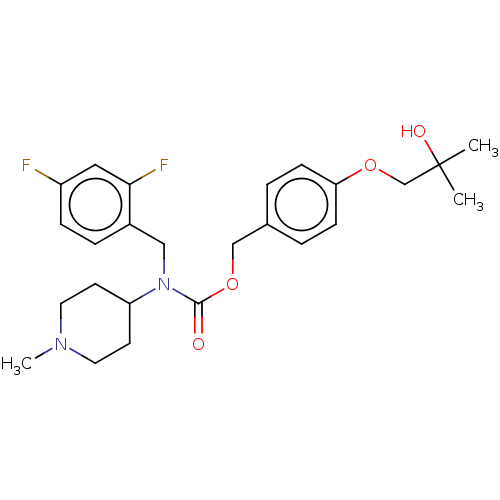
(US11440884, Example 29)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OCC(C)(C)O)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50320375
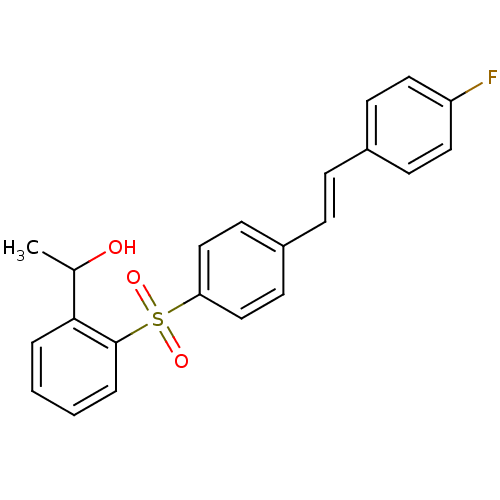
((E)-1-(2-(4-(4-fluorostyryl)phenylsulfonyl)phenyl)...)Show SMILES CC(O)c1ccccc1S(=O)(=O)c1ccc(\C=C\c2ccc(F)cc2)cc1 Show InChI InChI=1S/C22H19FO3S/c1-16(24)21-4-2-3-5-22(21)27(25,26)20-14-10-18(11-15-20)7-6-17-8-12-19(23)13-9-17/h2-16,24H,1H3/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM85034

(BW-501 | BW-501C | BW501)Show InChI InChI=1S/C17H20ClN3O/c18-15-9-4-5-10-16(15)22-12-6-11-20-17(19)13-21-14-7-2-1-3-8-14/h1-5,7-10,21H,6,11-13H2,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research Foundation
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 124: 57-73 (1996)
Article DOI: 10.1007/bf02245606
BindingDB Entry DOI: 10.7270/Q2610XV6 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570567
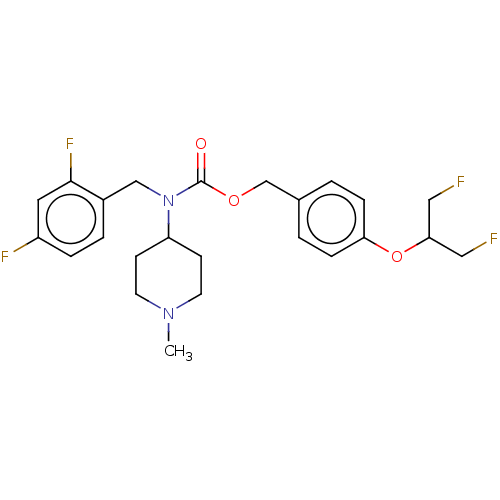
(US11440884, Example 28)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC(CF)CF)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803
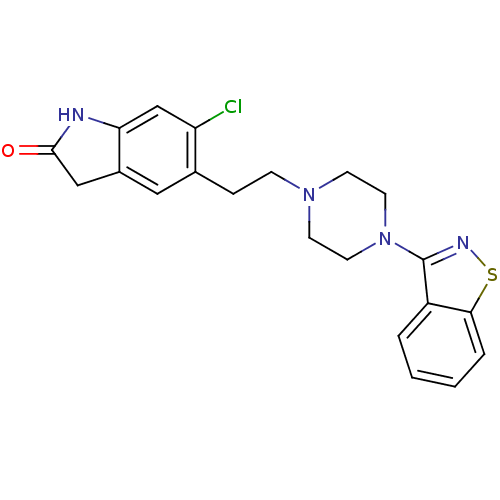
(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50231445
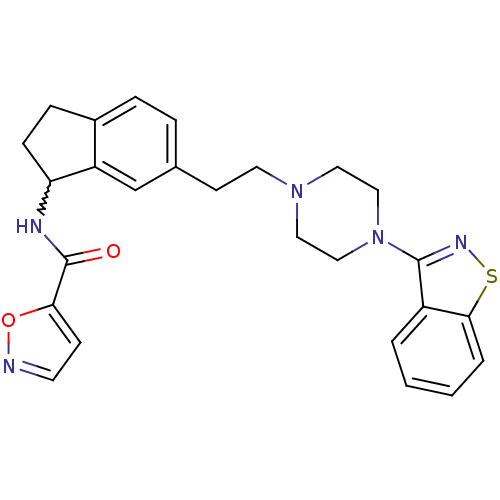
(CHEMBL251834 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...)Show SMILES O=C(NC1CCc2ccc(CCN3CCN(CC3)c3nsc4ccccc34)cc12)c1ccno1 |w:3.2| Show InChI InChI=1S/C26H27N5O2S/c32-26(23-9-11-27-33-23)28-22-8-7-19-6-5-18(17-21(19)22)10-12-30-13-15-31(16-14-30)25-20-3-1-2-4-24(20)34-29-25/h1-6,9,11,17,22H,7-8,10,12-16H2,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50095041
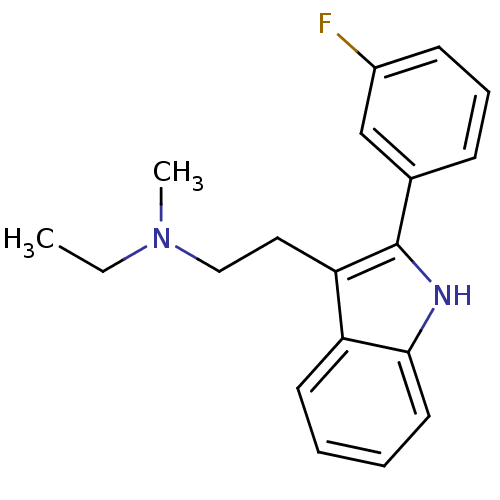
(CHEMBL328595 | Ethyl-{2-[2-(3-fluoro-phenyl)-1H-in...)Show InChI InChI=1S/C19H21FN2/c1-3-22(2)12-11-17-16-9-4-5-10-18(16)21-19(17)14-7-6-8-15(20)13-14/h4-10,13,21H,3,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacent of [H]-ketanserin from CHO cells expressing human 5-hydroxytryptamine 2A receptor. |
Bioorg Med Chem Lett 10: 2697-9 (2000)
BindingDB Entry DOI: 10.7270/Q27943XR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0830 | -12.8 | n/a | n/a | n/a | n/a | n/a | n/a | 4 |
ACADIA Pharmaceuticals Inc.
| Assay Description
For the membrane binding, NIH-3T3 cells were grown to 70% confluence in 15-cm2 dishes and transfected with 10 ug of receptor plasmid DNA using Polyfe... |
J Pharmacol Exp Ther 317: 910-8 (2006)
Article DOI: 10.1124/jpet.105.097006
BindingDB Entry DOI: 10.7270/Q269728N |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50231453
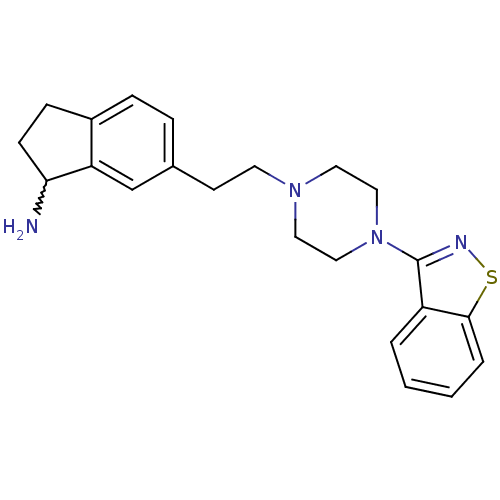
(6-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES NC1CCc2ccc(CCN3CCN(CC3)c3nsc4ccccc34)cc12 |w:1.0| Show InChI InChI=1S/C22H26N4S/c23-20-8-7-17-6-5-16(15-19(17)20)9-10-25-11-13-26(14-12-25)22-18-3-1-2-4-21(18)27-24-22/h1-6,15,20H,7-14,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099255
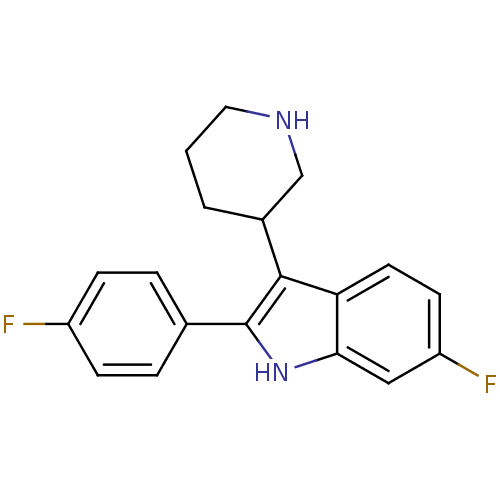
(6-Fluoro-2-(4-fluoro-phenyl)-3-piperidin-3-yl-1H-i...)Show InChI InChI=1S/C19H18F2N2/c20-14-5-3-12(4-6-14)19-18(13-2-1-9-22-11-13)16-8-7-15(21)10-17(16)23-19/h3-8,10,13,22-23H,1-2,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50343274
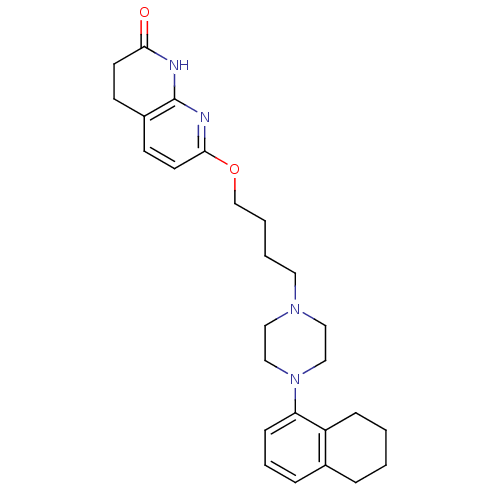
(7-(4-(4-(5,6,7,8-tetrahydronaphthalen-1-yl)piperaz...)Show SMILES O=C1CCc2ccc(OCCCCN3CCN(CC3)c3cccc4CCCCc34)nc2N1 Show InChI InChI=1S/C26H34N4O2/c31-24-12-10-21-11-13-25(28-26(21)27-24)32-19-4-3-14-29-15-17-30(18-16-29)23-9-5-7-20-6-1-2-8-22(20)23/h5,7,9,11,13H,1-4,6,8,10,12,14-19H2,(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT2A receptor |
Bioorg Med Chem Lett 21: 2621-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.059
BindingDB Entry DOI: 10.7270/Q29K4BJ3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099275
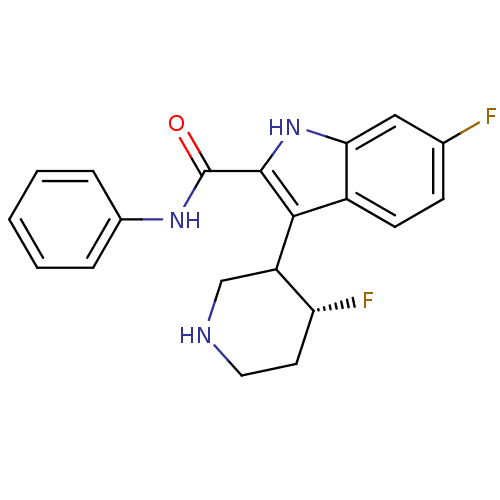
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-1H-indole-2-c...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H19F2N3O/c21-12-6-7-14-17(10-12)25-19(18(14)15-11-23-9-8-16(15)22)20(26)24-13-4-2-1-3-5-13/h1-7,10,15-16,23,25H,8-9,11H2,(H,24,26)/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50297306
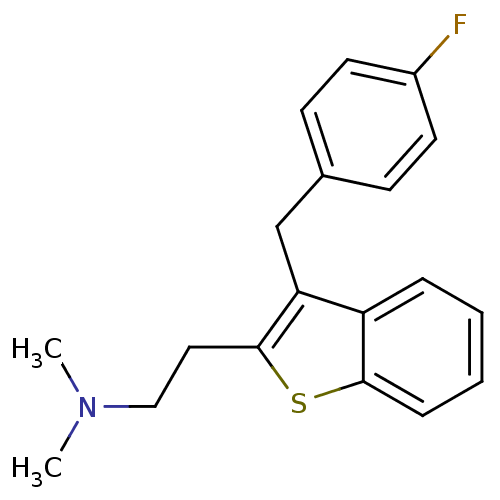
(CHEMBL540982 | {2-[3-(4-Fluoro-benzyl)-benzo[b]thi...)Show InChI InChI=1S/C19H20FNS/c1-21(2)12-11-19-17(13-14-7-9-15(20)10-8-14)16-5-3-4-6-18(16)22-19/h3-10H,11-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in HEK293 Flp-In cells by liquid scintillation counting |
J Med Chem 52: 5307-10 (2009)
Article DOI: 10.1021/jm900933k
BindingDB Entry DOI: 10.7270/Q2057G0S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50547388
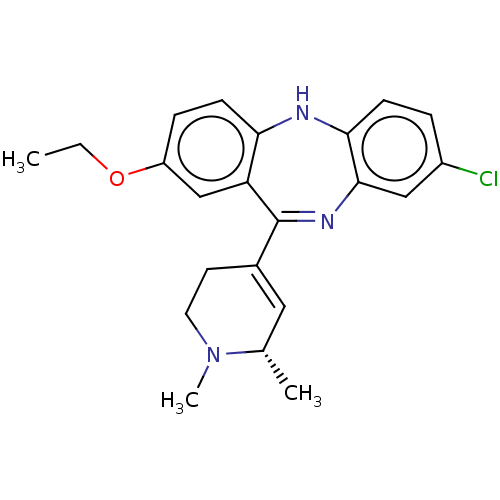
(CHEMBL4758966)Show SMILES CCOc1ccc2Nc3ccc(Cl)cc3N=C(C3=C[C@H](C)N(C)CC3)c2c1 |r,t:16,18| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50547385
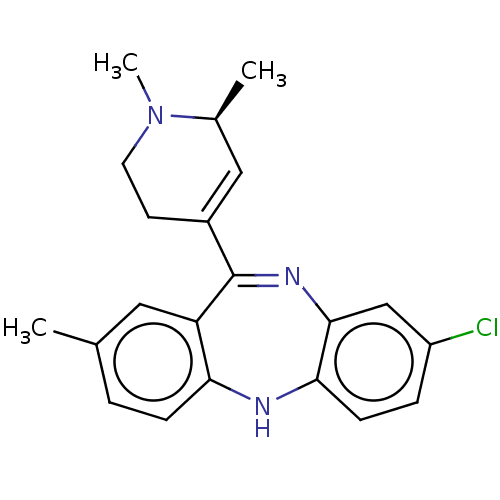
(CHEMBL4745124)Show SMILES C[C@H]1C=C(CCN1C)C1=Nc2cc(Cl)ccc2Nc2ccc(C)cc12 |r,c:2,t:9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 5HT2A receptor |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127563
BindingDB Entry DOI: 10.7270/Q26113Z0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570517
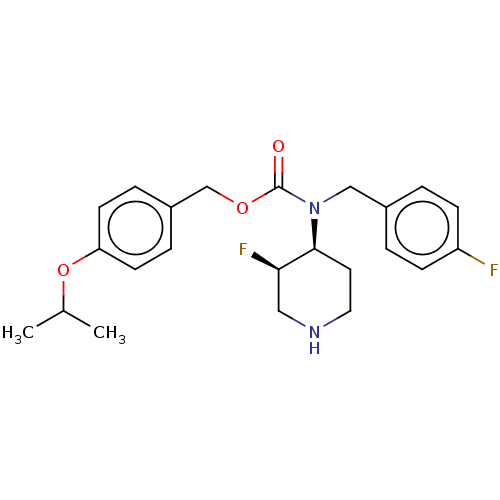
(US11440884, Example 3a | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@H]2CCNC[C@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50038677
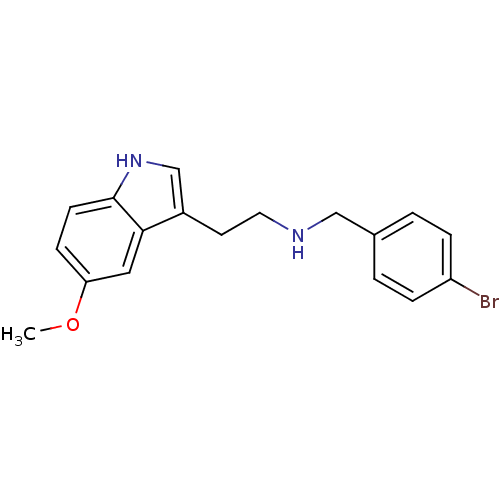
((4-Bromo-benzyl)-[2-(5-methoxy-1H-indol-3-yl)-ethy...)Show InChI InChI=1S/C18H19BrN2O/c1-22-16-6-7-18-17(10-16)14(12-21-18)8-9-20-11-13-2-4-15(19)5-3-13/h2-7,10,12,20-21H,8-9,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Affinity against 5-hydroxytryptamine 2A receptor (D) labeled with [125I]-DOI. |
J Med Chem 37: 1929-35 (1994)
BindingDB Entry DOI: 10.7270/Q2GQ6WT0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570525
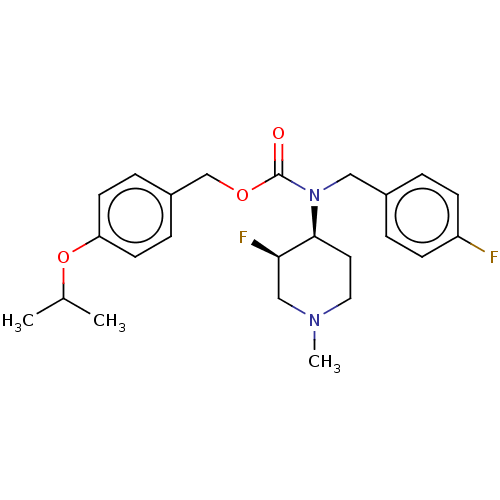
( [4-(propan-2-yloxy)phenyl]methyl N-[(3R,4S)-3-flu...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@H]2CCN(C)C[C@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570526
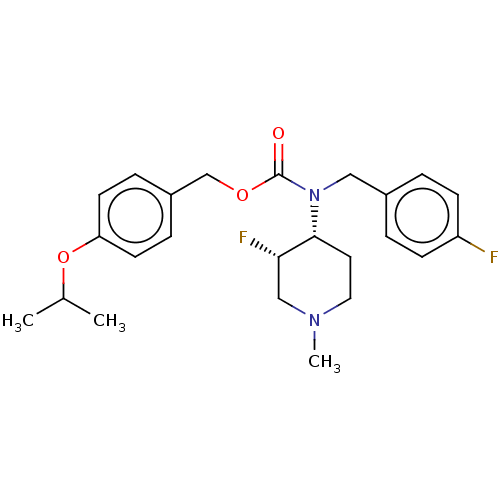
(US11440884, Example 4b | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@@H]2CCN(C)C[C@@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570571
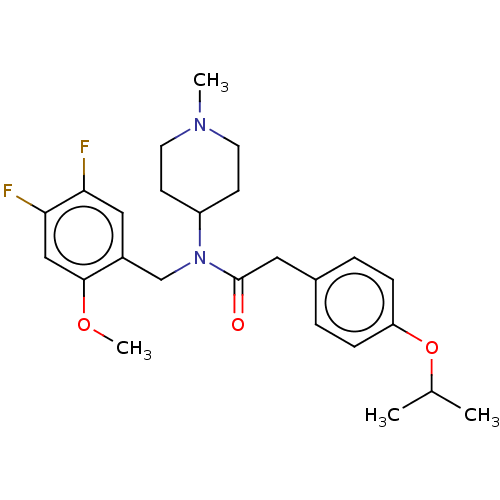
(US11440884, Example 32)Show SMILES COc1cc(F)c(F)cc1CN(C1CCN(C)CC1)C(=O)Cc1ccc(OC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570574
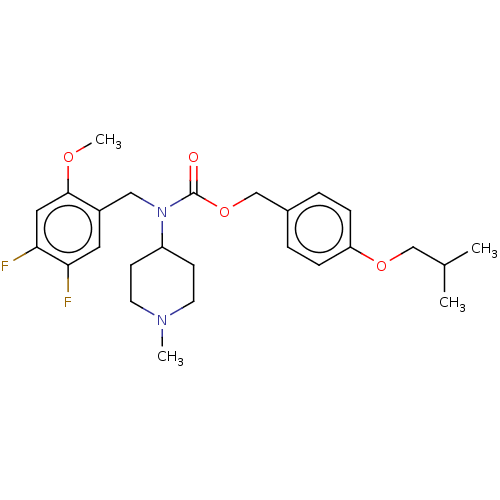
(US11440884, Example 35)Show SMILES COc1cc(F)c(F)cc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OCC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570602
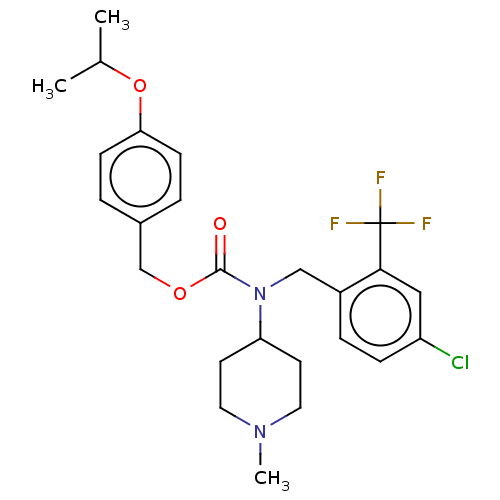
(US11440884, Example 54)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(Cl)cc2C(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570603
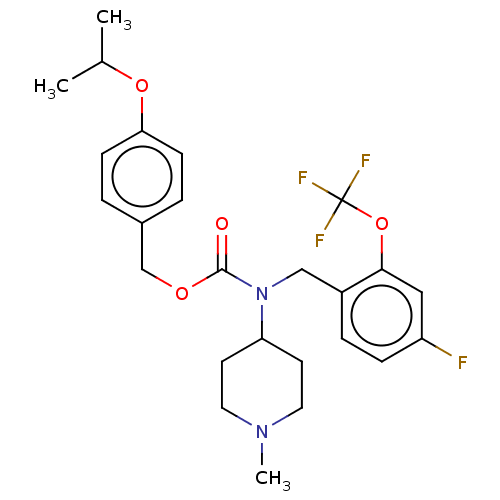
(US11440884, Example 55)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2OC(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570616
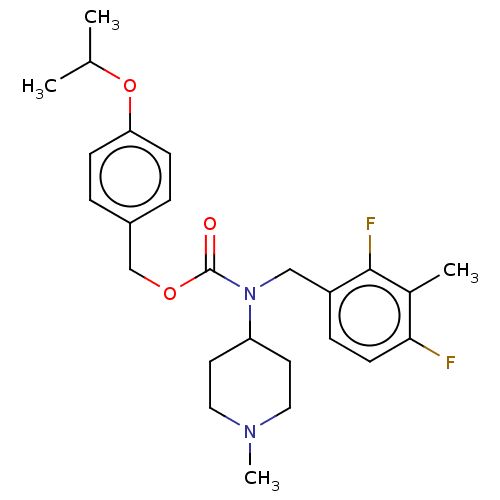
(US11440884, Example 67)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)c(C)c2F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570619
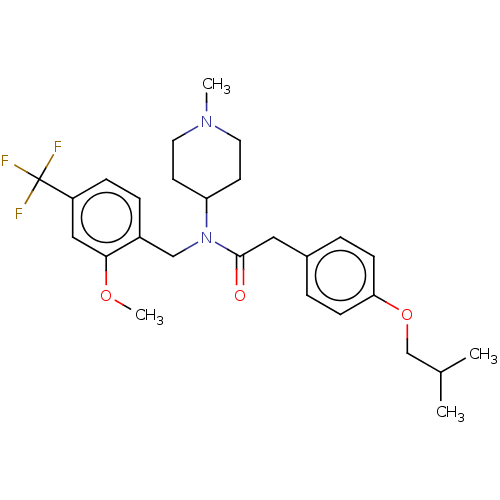
(US11440884, Example 70)Show SMILES COc1cc(ccc1CN(C1CCN(C)CC1)C(=O)Cc1ccc(OCC(C)C)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570621
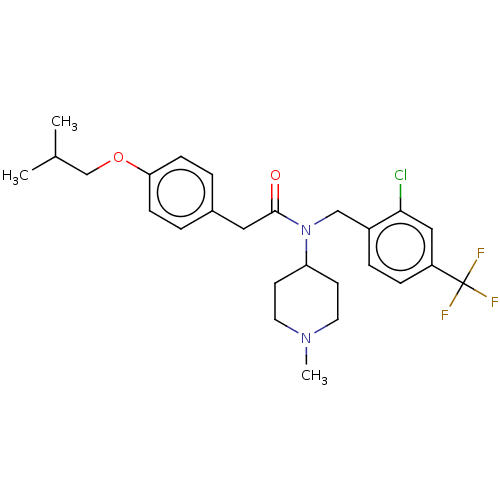
(US11440884, Example 71)Show SMILES CC(C)COc1ccc(CC(=O)N(Cc2ccc(cc2Cl)C(F)(F)F)C2CCN(C)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570622
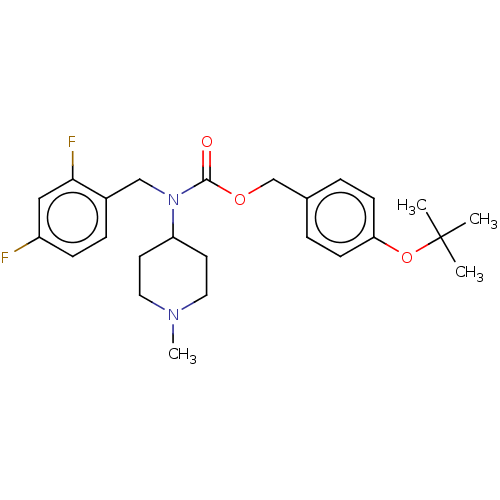
(US11440884, Example 72)Show SMILES CN1CCC(CC1)N(Cc1ccc(F)cc1F)C(=O)OCc1ccc(OC(C)(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570627
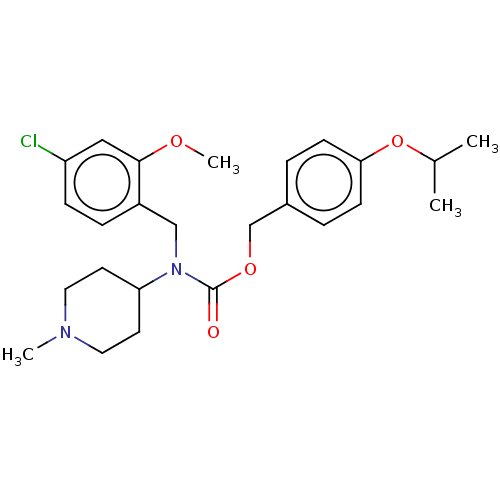
(US11440884, Example 76)Show SMILES COc1cc(Cl)ccc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OC(C)C)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570628

(US11440884, Example 77)Show SMILES COc1cc(ccc1CN(C1CCN(C)CC1)C(=O)OCc1ccc(OC(C)C)cc1)C(F)(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50231452

(CHEMBL253439 | N-(6-(2-(4-(benzo[d]isothiazol-3-yl...)Show SMILES Clc1ccc(cc1)C(=O)NC1CCc2ccc(CCN3CCN(CC3)c3nsc4ccccc34)cc12 |w:10.10| Show InChI InChI=1S/C29H29ClN4OS/c30-23-10-7-22(8-11-23)29(35)31-26-12-9-21-6-5-20(19-25(21)26)13-14-33-15-17-34(18-16-33)28-24-3-1-2-4-27(24)36-32-28/h1-8,10-11,19,26H,9,12-18H2,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in Swiss 3T3 cells |
Bioorg Med Chem Lett 18: 489-93 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.106
BindingDB Entry DOI: 10.7270/Q2611023 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM78940
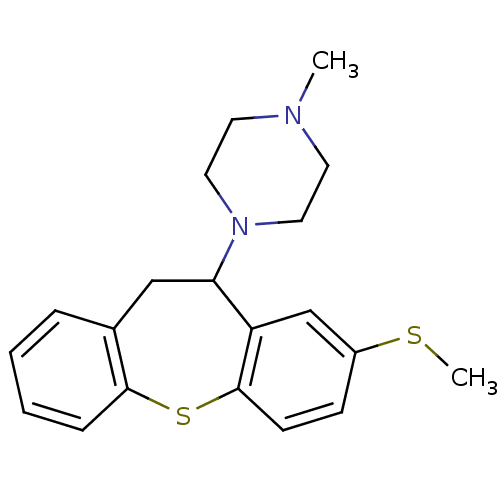
(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50014997
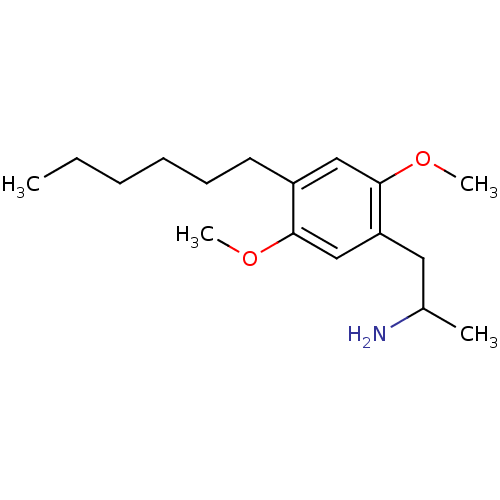
(1-(4-hexyl-2,5-dimethoxyphenyl)propan-2-amine | 2-...)Show InChI InChI=1S/C17H29NO2/c1-5-6-7-8-9-14-11-17(20-4)15(10-13(2)18)12-16(14)19-3/h11-13H,5-10,18H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 359: 1-6 (1999)
Article DOI: 10.1007/pl00005315
BindingDB Entry DOI: 10.7270/Q2862F09 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM570524
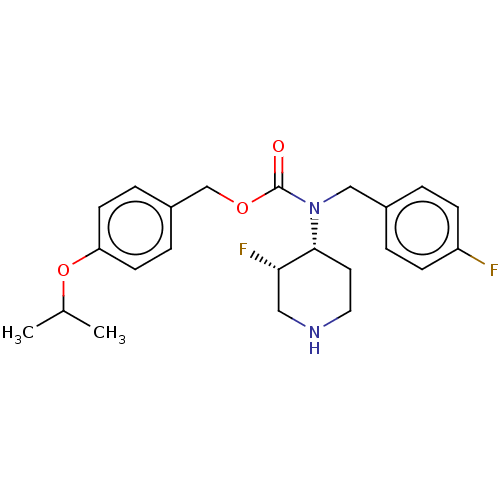
(US11440884, Example 3b | [4-(propan-2-yloxy)phenyl...)Show SMILES CC(C)Oc1ccc(COC(=O)N(Cc2ccc(F)cc2)[C@@H]2CCNC[C@@H]2F)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
R-SAT's were generally performed with 50 ng/well of receptor and 20 ng/well of β-galactosidase plasmid DNA. All receptor constructs used wer... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2ZC864D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM458582
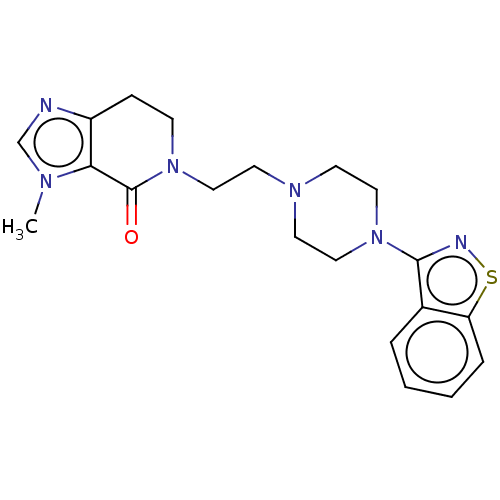
(US10745401, Example 56 | US11466007, Example 56)Show SMILES Cn1cnc2CCN(CCN3CCN(CC3)c3nsc4ccccc34)C(=O)c12 Show InChI InChI=1S/C20H24N6OS/c1-23-14-21-16-6-7-26(20(27)18(16)23)13-10-24-8-11-25(12-9-24)19-15-4-2-3-5-17(15)28-22-19/h2-5,14H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q22V2KB4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099259
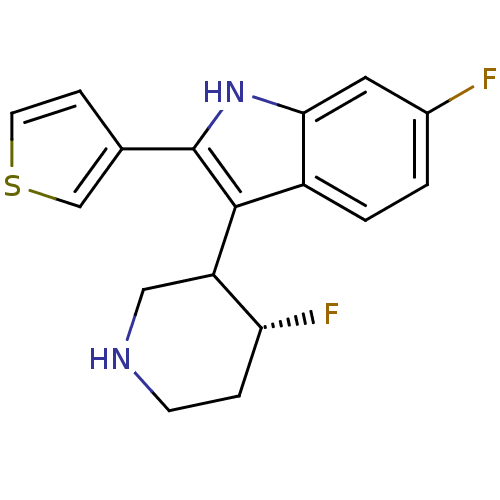
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-thiophen-3-...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccsc1 Show InChI InChI=1S/C17H16F2N2S/c18-11-1-2-12-15(7-11)21-17(10-4-6-22-9-10)16(12)13-8-20-5-3-14(13)19/h1-2,4,6-7,9,13-14,20-21H,3,5,8H2/t13?,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM458582

(US10745401, Example 56 | US11466007, Example 56)Show SMILES Cn1cnc2CCN(CCN3CCN(CC3)c3nsc4ccccc34)C(=O)c12 Show InChI InChI=1S/C20H24N6OS/c1-23-14-21-16-6-7-26(20(27)18(16)23)13-10-24-8-11-25(12-9-24)19-15-4-2-3-5-17(15)28-22-19/h2-5,14H,6-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD.
US Patent
| Assay Description
Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... |
US Patent US10745401 (2020)
BindingDB Entry DOI: 10.7270/Q2D221P5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099269
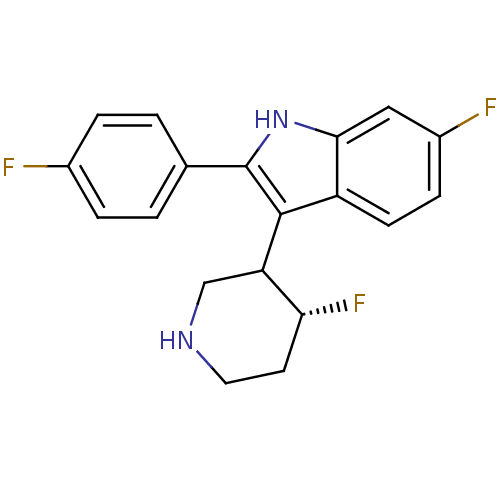
(6-Fluoro-2-(4-fluoro-phenyl)-3-(4-fluoro-piperidin...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccc(F)cc1 Show InChI InChI=1S/C19H17F3N2/c20-12-3-1-11(2-4-12)19-18(15-10-23-8-7-16(15)22)14-6-5-13(21)9-17(14)24-19/h1-6,9,15-16,23-24H,7-8,10H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50034043
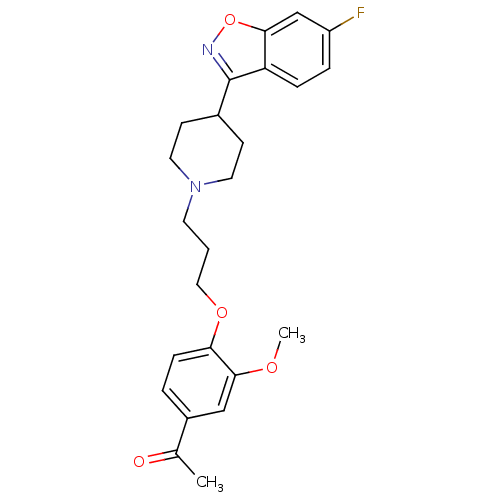
(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Mol Psychiatry 3: 123-34 (1998)
Article DOI: 10.1038/sj.mp.4000336
BindingDB Entry DOI: 10.7270/Q2G15ZCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data