

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50601469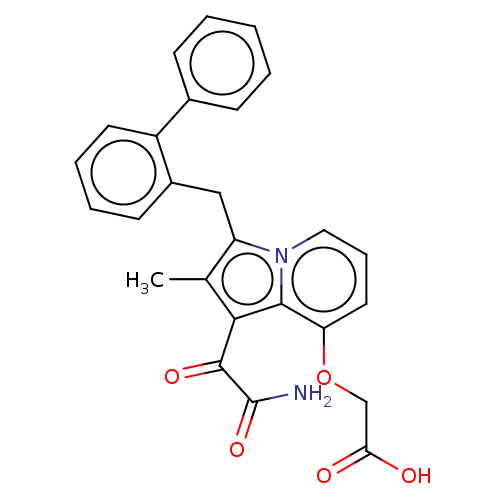 (CHEMBL5172164) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01808 BindingDB Entry DOI: 10.7270/Q2SF316G | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50263002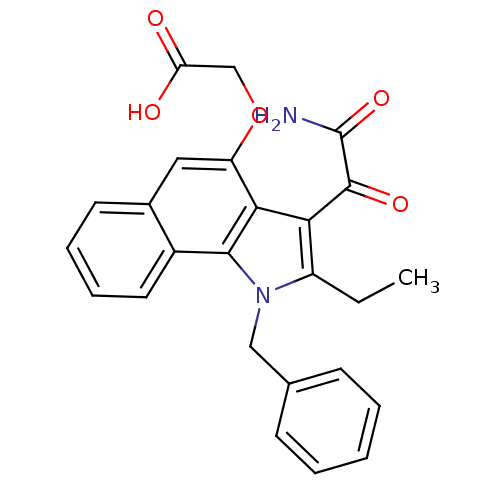 ((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055391 (2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50053137 ((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50262842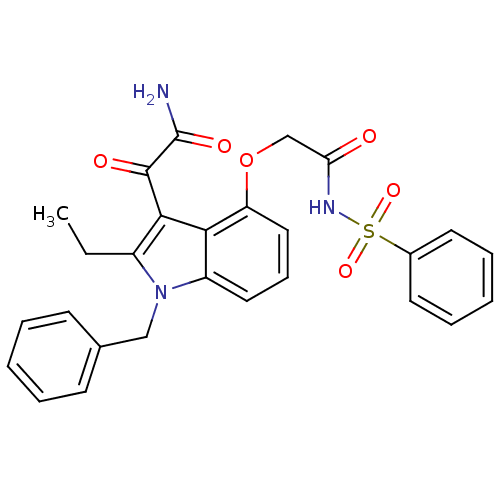 (Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274336 (1-{3-Dodecanoyl-2,4,6-trihydroxy-5-[7-hydroxy-2-(4...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-5 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate p... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50263000 (2-(1-benzyl-2-ethyl-1H-6,7-benzoindol-4-yloxy)-N-(...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458608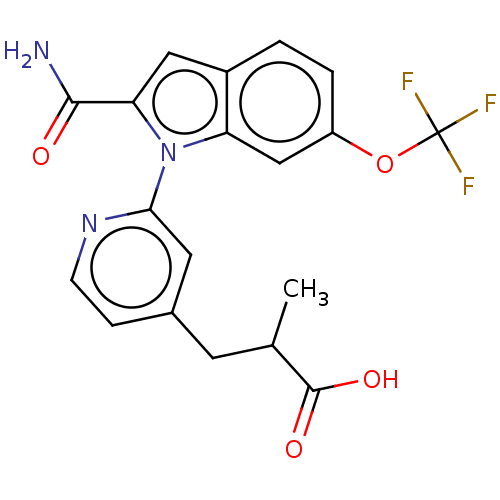 (CHEMBL4205008) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458608 (CHEMBL4205008) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055371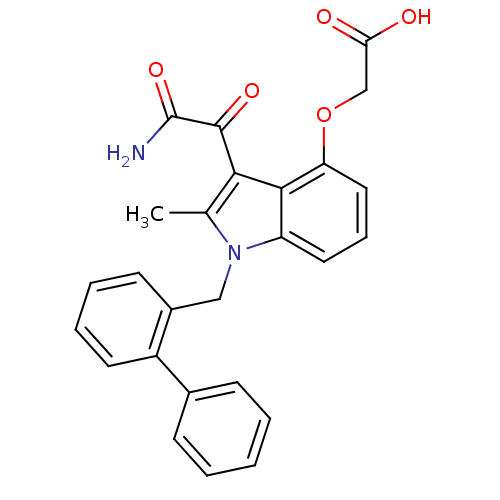 ((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50333788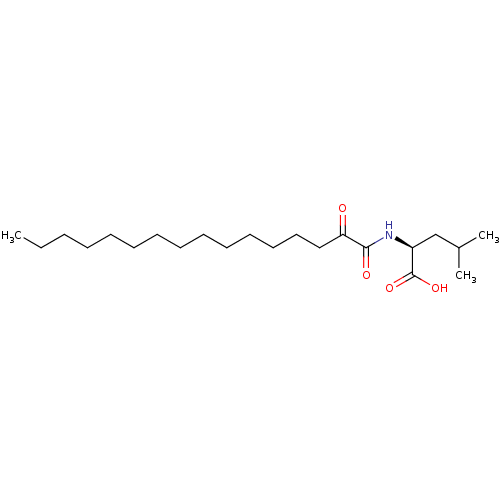 ((S)-4-Methyl-2-(2-oxohexadecanamido)pentanoic acid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human group 5 sPLA2 by fluorescence assay | Bioorg Med Chem 19: 735-43 (2011) Article DOI: 10.1016/j.bmc.2010.12.030 BindingDB Entry DOI: 10.7270/Q2JM29VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50186585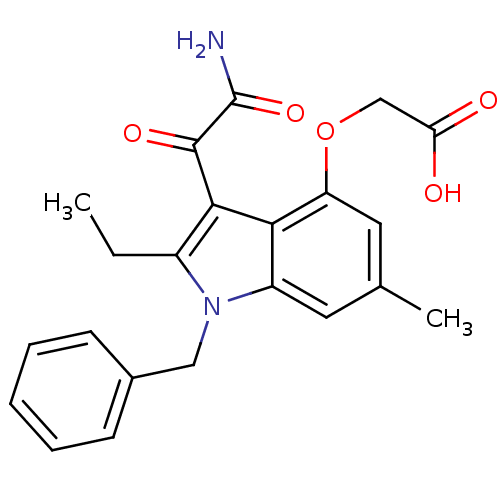 (2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human recombinant sPLA2 G5 | J Med Chem 49: 2858-60 (2006) Article DOI: 10.1021/jm060136t BindingDB Entry DOI: 10.7270/Q26W99PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human recombinant sPLA2 G5 | J Med Chem 49: 2858-60 (2006) Article DOI: 10.1021/jm060136t BindingDB Entry DOI: 10.7270/Q26W99PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458612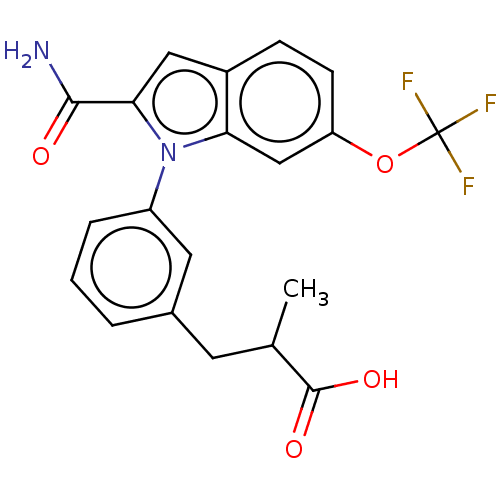 (CHEMBL4214052) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458606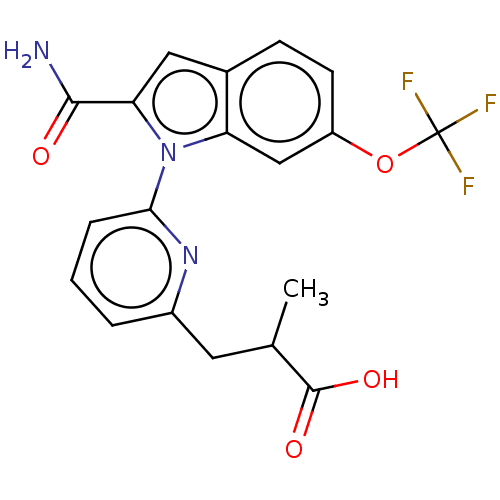 (CHEMBL4205511) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274337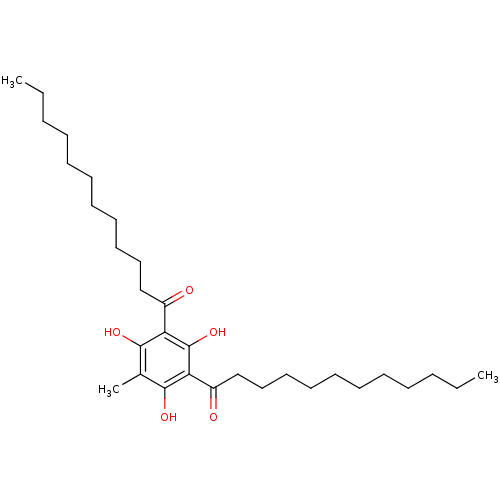 (1-(3-Dodecanoyl-2,4,6-trihydroxy-5-methyl-phenyl)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50206911 ((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz | Assay Description Briefly, sn-2ester bond of the substrate 1,2-bis(heptanoylthio)-glycerophosphocholine was hydrolyzed by PLA2-V followed by the exposure of free thiol... | Chem Biol Drug Des 85: 729-42 (2015) Article DOI: 10.1111/cbdd.12457 BindingDB Entry DOI: 10.7270/Q26W98TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50206920 ((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458609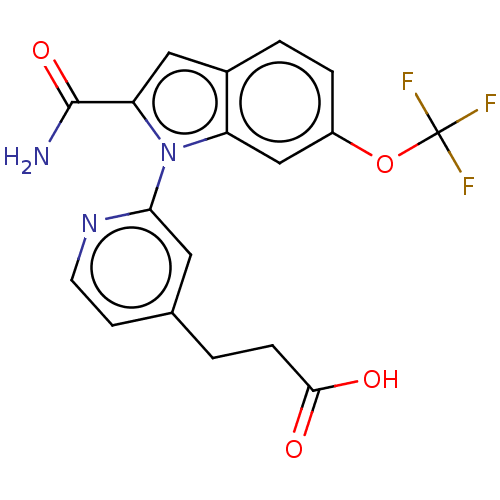 (CHEMBL4213094) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055383 ((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human recombinant sPLA2 G5 | J Med Chem 49: 2858-60 (2006) Article DOI: 10.1021/jm060136t BindingDB Entry DOI: 10.7270/Q26W99PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50186586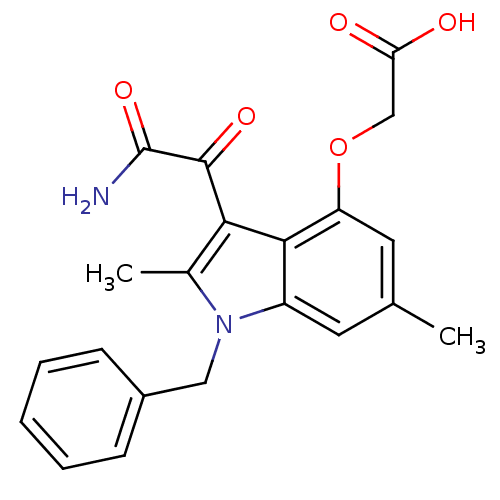 (2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2,6-dimethyl-1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human recombinant sPLA2 G5 | J Med Chem 49: 2858-60 (2006) Article DOI: 10.1021/jm060136t BindingDB Entry DOI: 10.7270/Q26W99PJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50206910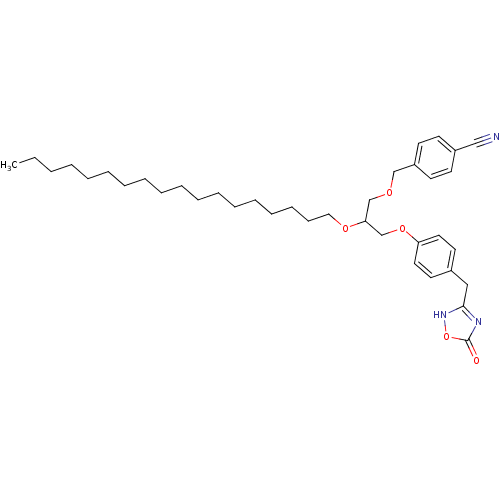 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50591714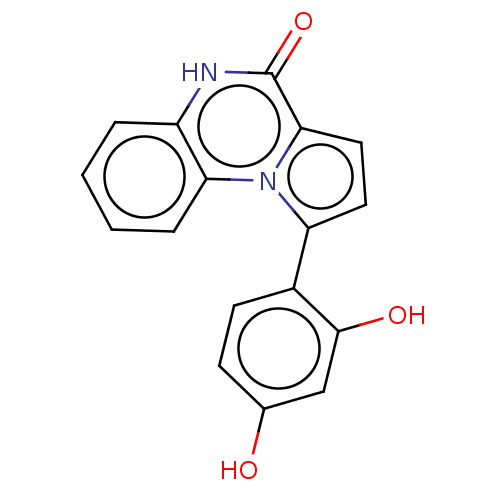 (CHEMBL5200953) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114085 BindingDB Entry DOI: 10.7270/Q2XW4PS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50149981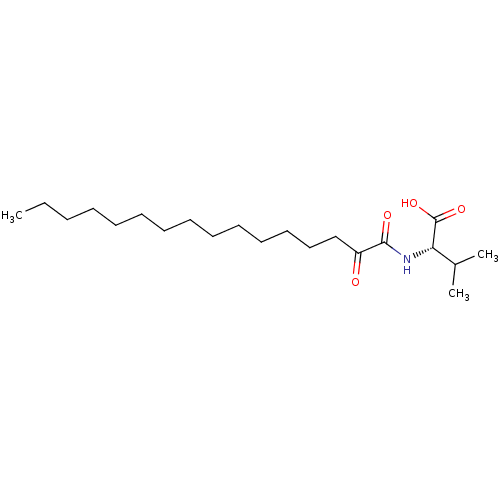 (CHEMBL3769794) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens Curated by ChEMBL | Assay Description Inhibition of human group 5 secreted phospholipase A2 by fluorescence assay | Bioorg Med Chem 24: 1683-95 (2016) Article DOI: 10.1016/j.bmc.2016.02.040 BindingDB Entry DOI: 10.7270/Q2JQ12WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50591712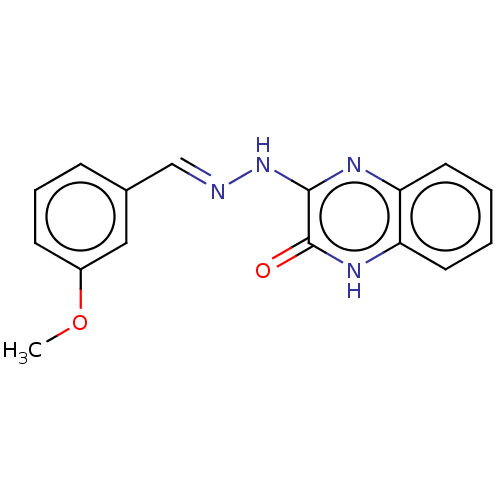 (CHEMBL5194852) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114085 BindingDB Entry DOI: 10.7270/Q2XW4PS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50333789 ((R)-4-Methyl-2-(2-oxohexadecanamido)pentanoic acid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human group 5 sPLA2 by fluorescence assay | Bioorg Med Chem 19: 735-43 (2011) Article DOI: 10.1016/j.bmc.2010.12.030 BindingDB Entry DOI: 10.7270/Q2JM29VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50591713 (CHEMBL5207115) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.114085 BindingDB Entry DOI: 10.7270/Q2XW4PS2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274389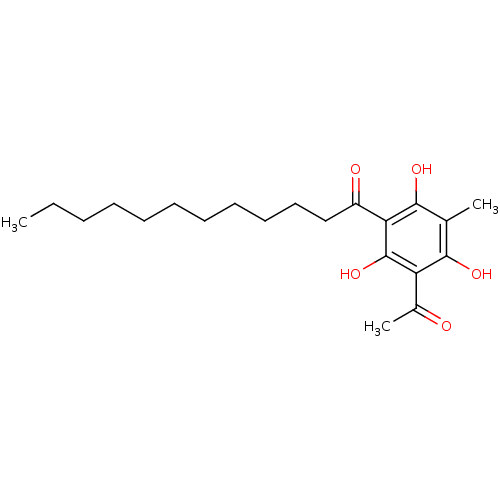 (1-(3-acetyl-2,4,6-trihydroxy-5-methylphenyl)dodeca...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274387 (1-(3-Acetyl-2,4,6-trihydroxy-5-methyl-phenyl)-etha...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274388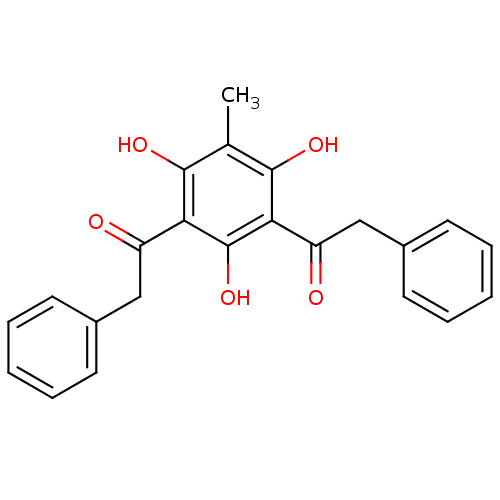 (2-Phenyl-1-(2,4,6-trihydroxy-3-methyl-5-phenylacet...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274441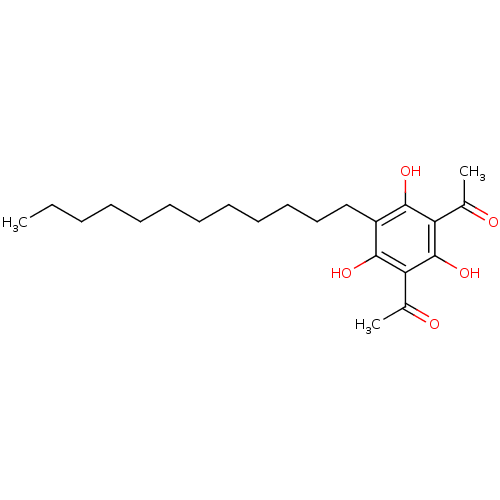 (1-(3-Acetyl-5-dodecyl-2,4,6-trihydroxy-phenyl)-eth...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50256011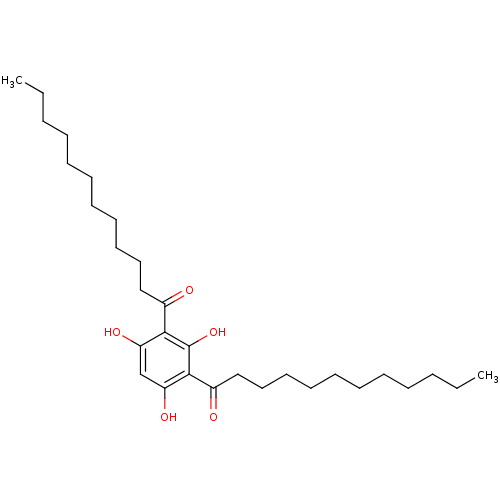 (1,1'-(2,4,6-trihydroxy-1,3-phenylene)didodecan-1-o...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274390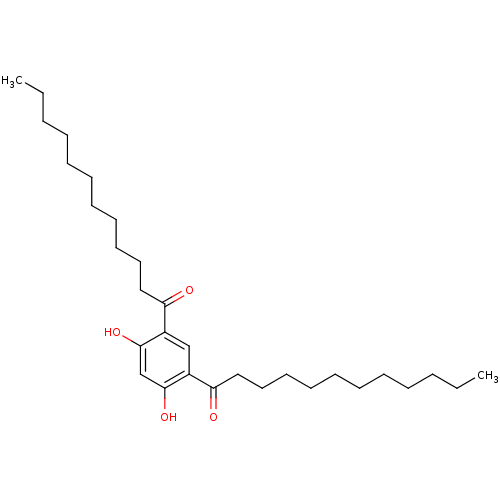 (1-(5-Dodecanoyl-2,4-dihydroxy-phenyl)-dodecan-1-on...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274391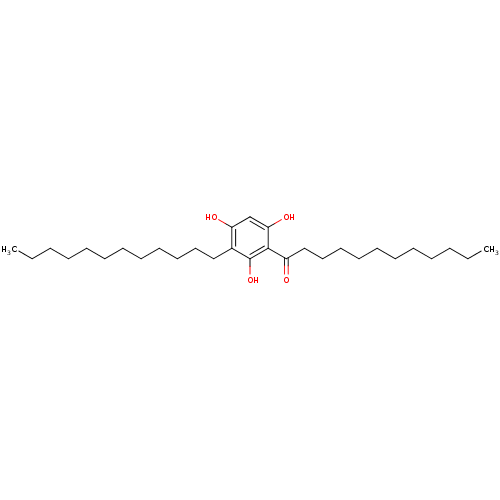 (1-(3-dodecyl-2,4,6-trihydroxyphenyl)dodecan-1-one ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50274442 (1-(3-Dodecanoyl-2-hydroxy-5-methyl-phenyl)-dodecan...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human sPLA2 group 5 | Bioorg Med Chem Lett 18: 5415-9 (2008) Article DOI: 10.1016/j.bmcl.2008.09.041 BindingDB Entry DOI: 10.7270/Q2CJ8DB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50263003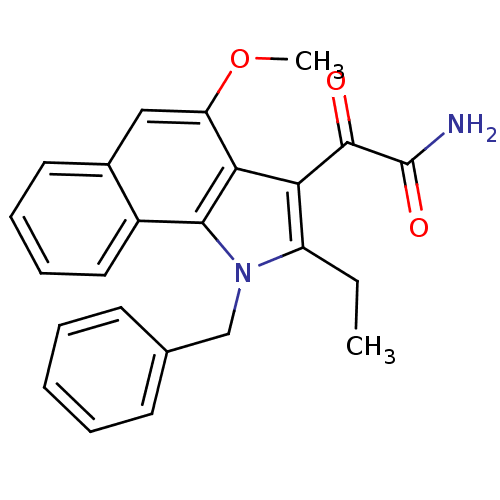 (2-(2-ethyl-3-(o-phenylbenzyl)-8-(2-oxopropoxy)indo...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50262804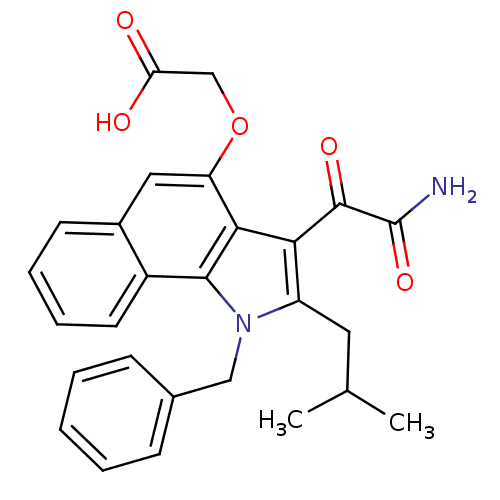 (2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-isobutyl-1H-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50263001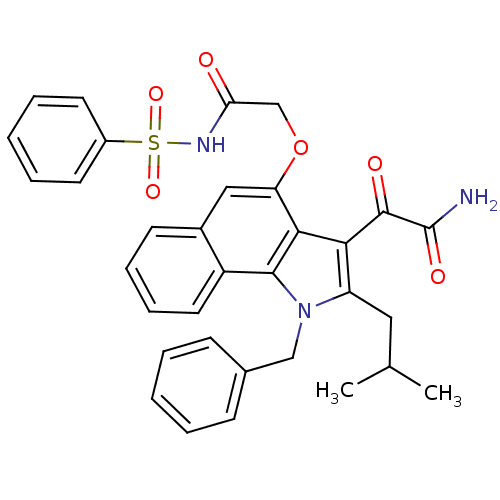 (2-(1-benzyl-2-isobutyl-1H-6,7-benzoindol-4-yloxy)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50262844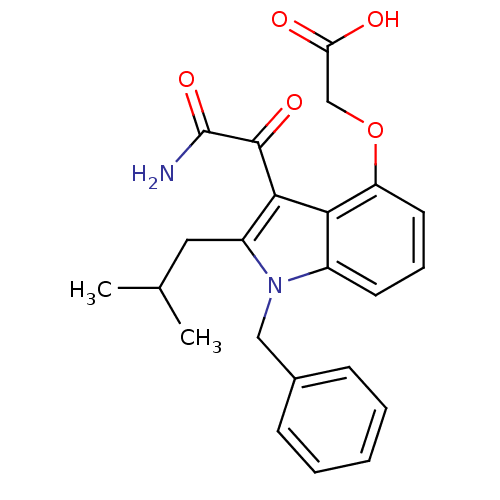 (2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-isobutyl-1H-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50262843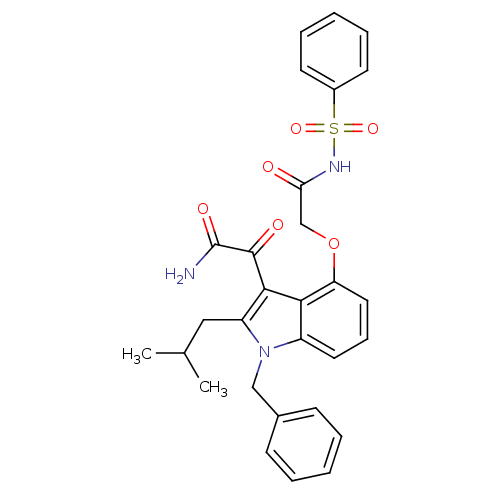 (Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50263053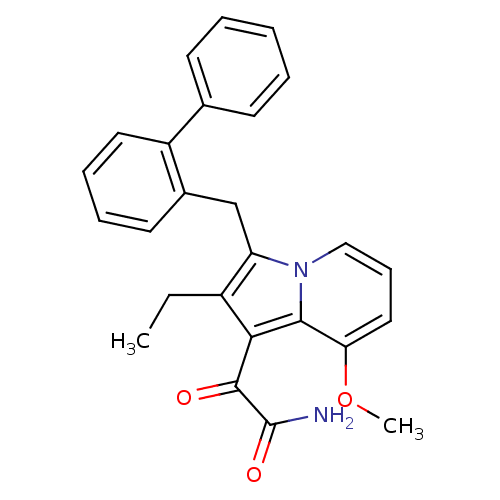 (2-(3-Biphenyl-2-ylmethyl-2-ethyl-8-methoxy-indoliz...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50263052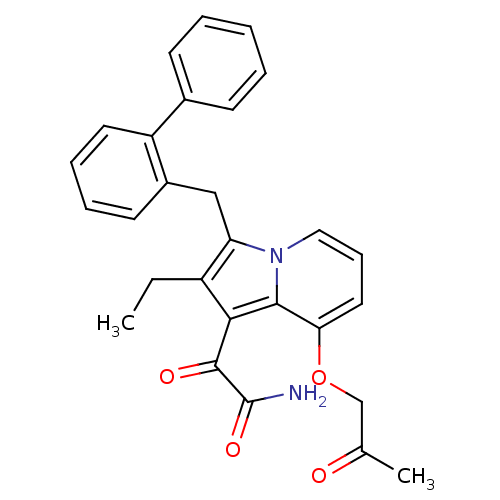 (2-[3-Biphenyl-2-ylmethyl-2-ethyl-8-(2-oxo-propoxy)...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50263051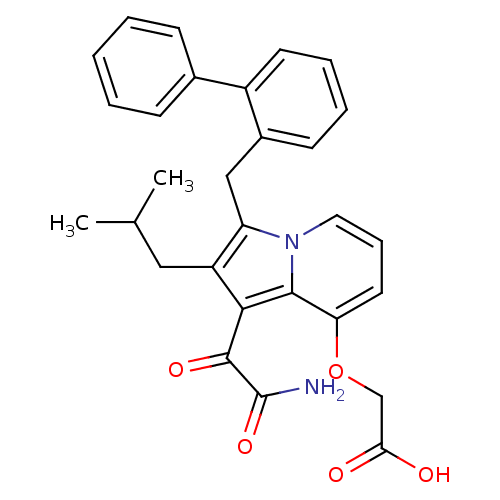 (2-(1-(2-amino-2-oxoacetyl)-2-(isobutyl)-3-(ophenyl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington Curated by ChEMBL | Assay Description Inhibition of human group2V phospholipase A2 fluorimetric assay | J Med Chem 51: 4708-14 (2008) Article DOI: 10.1021/jm800422v BindingDB Entry DOI: 10.7270/Q2571BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50333791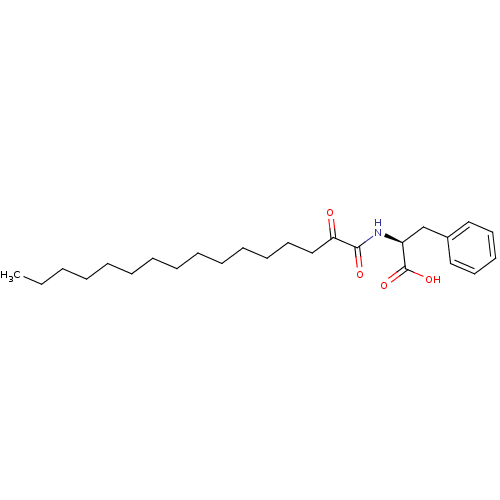 ((S)-2-(2-Oxohexadecanamido)-3-phenylpropanoic acid...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human group 5 sPLA2 by fluorescence assay | Bioorg Med Chem 19: 735-43 (2011) Article DOI: 10.1016/j.bmc.2010.12.030 BindingDB Entry DOI: 10.7270/Q2JM29VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50333790 ((S)-4-Methyl-2-palmitamidopentanoic acid | CHEMBL1...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens Curated by ChEMBL | Assay Description Inhibition of human group 5 sPLA2 by fluorescence assay | Bioorg Med Chem 19: 735-43 (2011) Article DOI: 10.1016/j.bmc.2010.12.030 BindingDB Entry DOI: 10.7270/Q2JM29VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50206908 ((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot Curated by ChEMBL | Assay Description Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay | J Med Chem 50: 1618-26 (2007) Article DOI: 10.1021/jm060082n BindingDB Entry DOI: 10.7270/Q2TQ6174 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458615 (CHEMBL4203027) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |