

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21513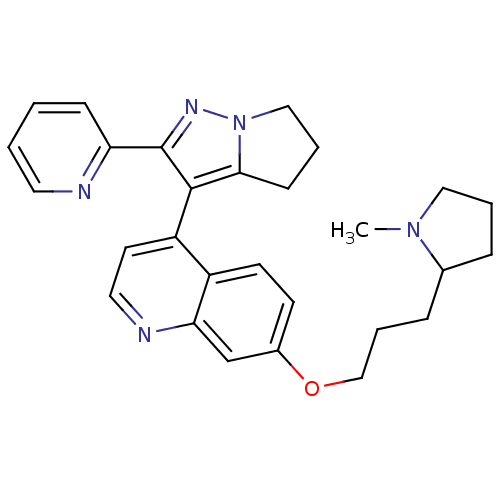 (7-[3-(1-methylpyrrolidin-2-yl)propoxy]-4-[2-(pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | 17 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21510 (Dihydropyrrolopyrazole, 16a | dimethyl[5-({4-[2-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | 24 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21506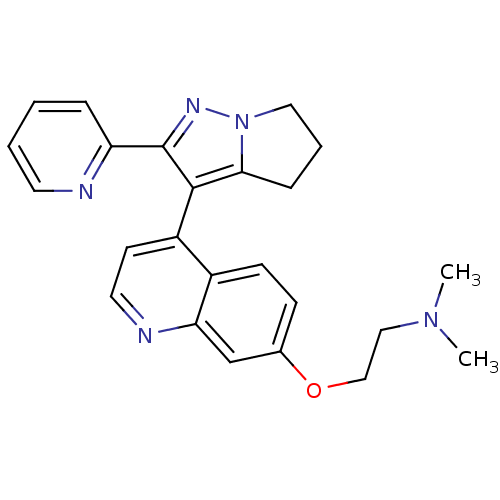 (Dihydropyrrolopyrazole, 15a | dimethyl[2-({4-[2-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | 29 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21508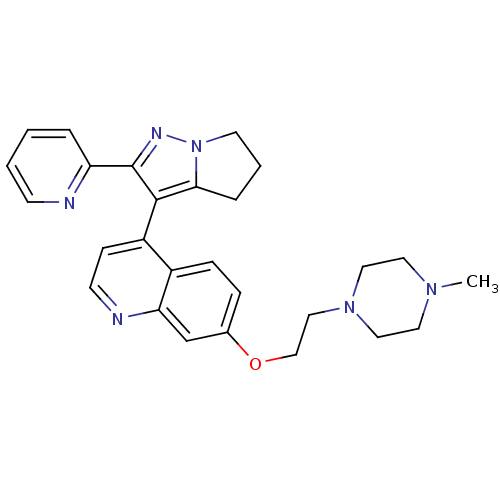 (7-[2-(4-methylpiperazin-1-yl)ethoxy]-4-[2-(pyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | 38 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21492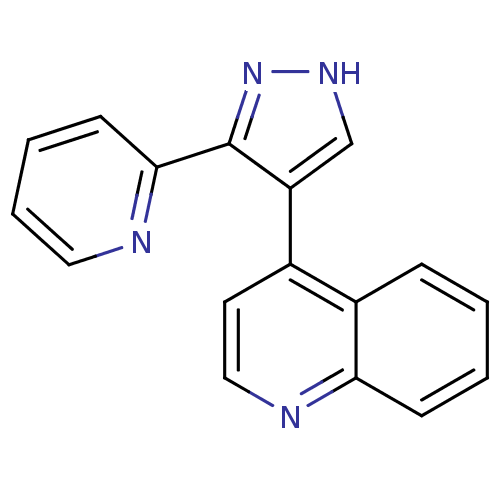 (4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline | CH...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 59 | n/a | 40 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21504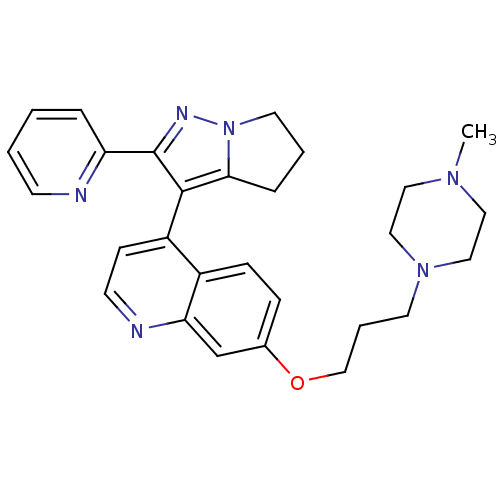 (7-[3-(4-methylpiperazin-1-yl)propoxy]-4-[2-(pyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 74 | n/a | 44 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21496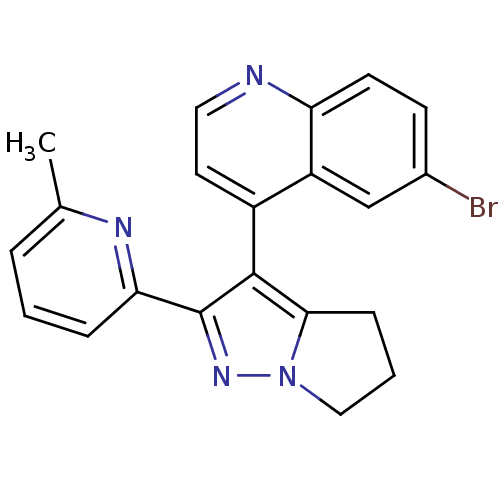 (6-bromo-4-[2-(6-methylpyridin-2-yl)-4H,5H,6H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 89 | n/a | 55 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21507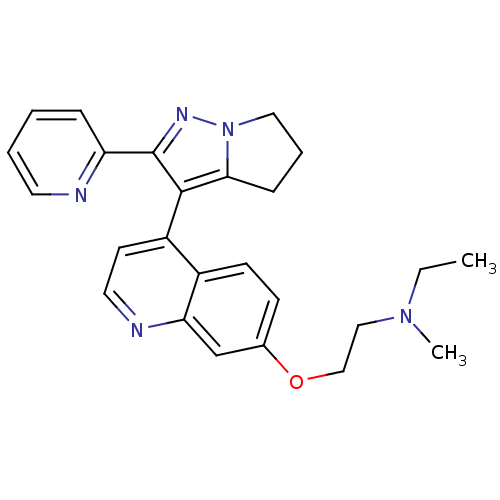 (Dihydropyrrolopyrazole, 15b | ethyl(methyl)[2-({4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | 61 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21505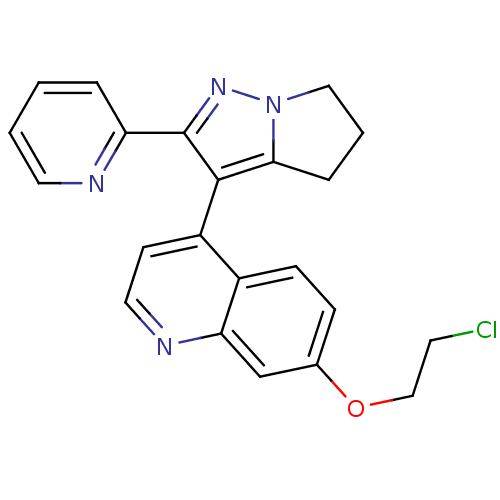 (7-(2-chloroethoxy)-4-[2-(pyridin-2-yl)-4H,5H,6H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | 66 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21512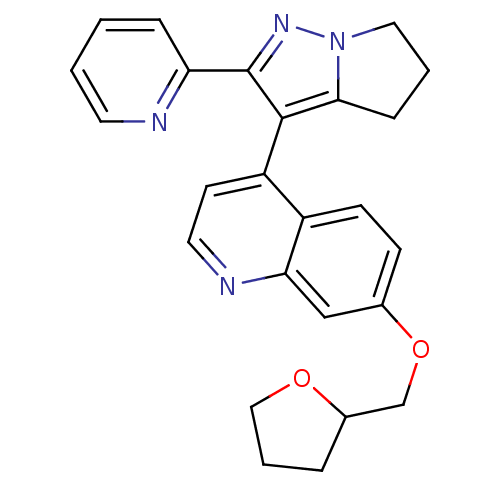 (7-(oxolan-2-ylmethoxy)-4-[2-(pyridin-2-yl)-4H,5H,6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | 80 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21509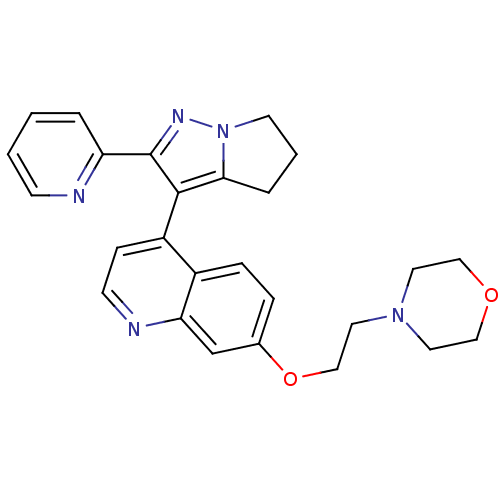 (4-[2-({4-[2-(pyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-a]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | 180 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21503 (4-[2-(pyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-a]pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | 200 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21497 (4-[2-(6-methylpyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | 201 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21511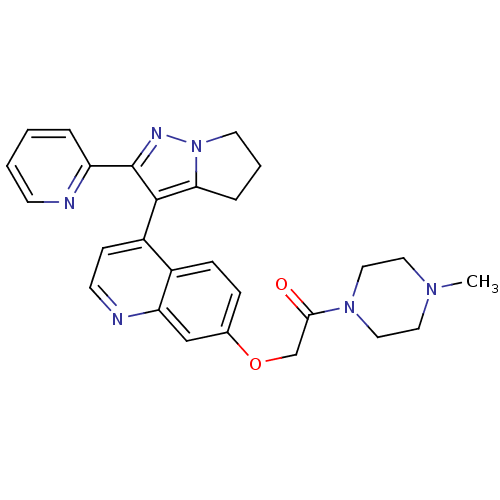 (1-(4-methylpiperazin-1-yl)-2-({4-[2-(pyridin-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | 260 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21498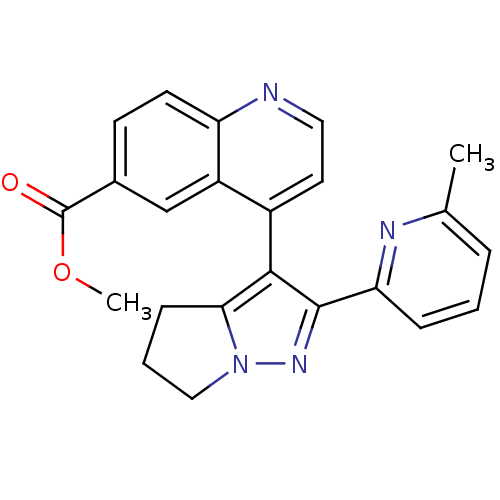 (Dihydropyrrolopyrazole, 8 | methyl 4-[2-(6-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 526 | n/a | 403 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21499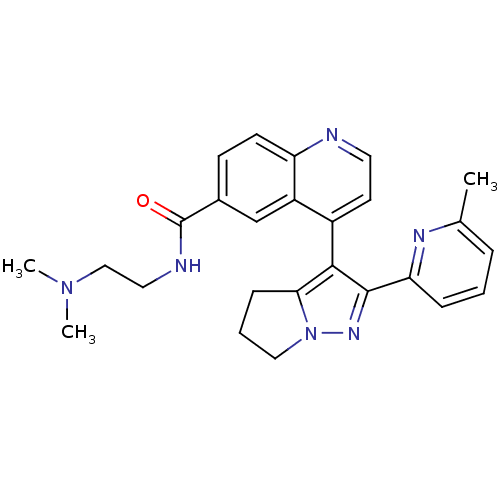 (Dihydropyrrolopyrazole, 9 | N-[2-(dimethylamino)et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 193 | n/a | 468 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21495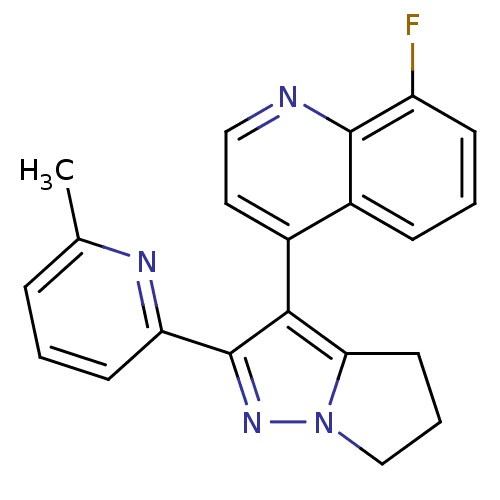 (8-fluoro-4-[2-(6-methylpyridin-2-yl)-4H,5H,6H-pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 928 | n/a | 951 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21494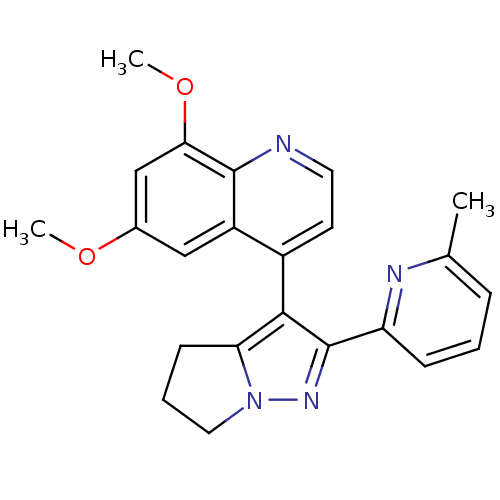 (6,8-dimethoxy-4-[2-(6-methylpyridin-2-yl)-4H,5H,6H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.81E+3 | n/a | 7.20E+3 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21517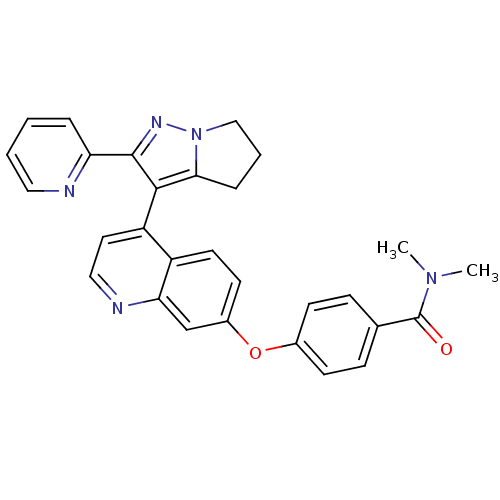 (Dihydropyrrolopyrazole, 22 | N,N-dimethyl-4-({4-[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | 1.50E+4 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21516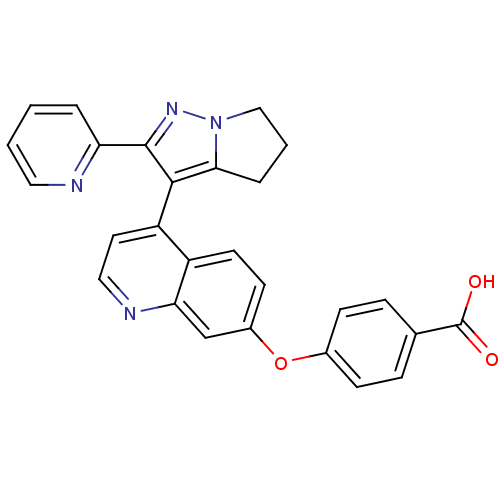 (4-({4-[2-(pyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-a]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | >2.00E+4 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21502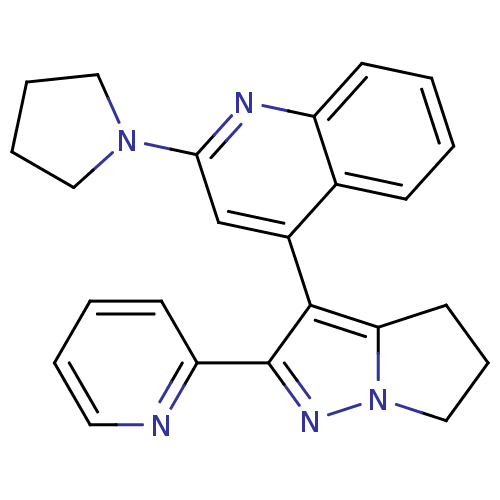 (4-[2-(pyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-a]pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | >2.00E+4 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21501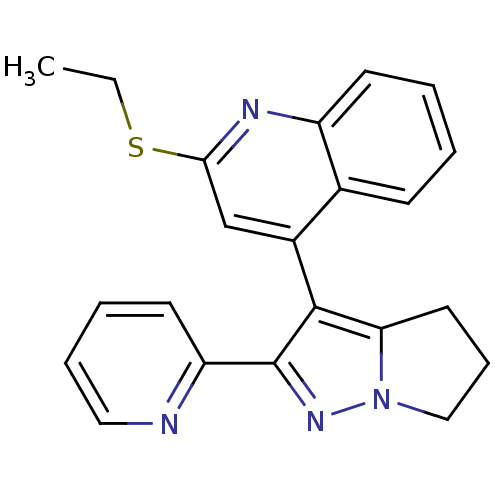 (2-(ethylsulfanyl)-4-[2-(pyridin-2-yl)-4H,5H,6H-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | >2.00E+4 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 [4-503,T204D] (Homo sapiens (Human)) | BDBM21493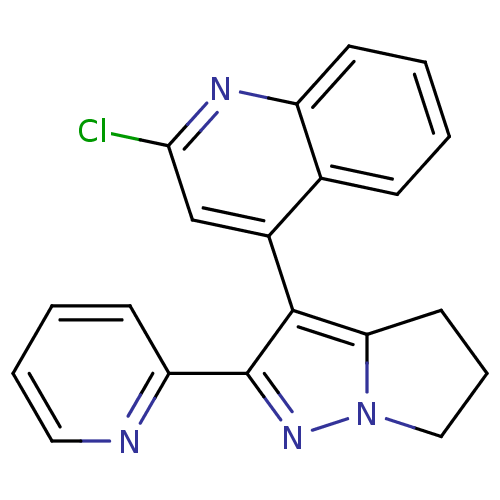 (2-chloro-4-[2-(pyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.98E+4 | n/a | >2.00E+4 | n/a | n/a | 7.5 | 30 |
Lilly Research Laboratories | Assay Description The kinase activity was assayed by the autophosphorylation reaction of ALK5 (T204D) in the presence of 4 uM ATP and [33P]-gamma-ATP. After incubation... | J Med Chem 51: 2302-2306 (2008) Article DOI: 10.1021/jm701199p BindingDB Entry DOI: 10.7270/Q2G15Z4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||