

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eosinophil peroxidase (Human) | BDBM50106515 (7-Benzyloxy-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human EPX bromination activity assessed as reduction in H2O2 catalyzed 3-bromo tyrosine formation from tyrosine and potassium bromide p... | ACS Med Chem Lett 9: 1175-1180 (2018) Article DOI: 10.1021/acsmedchemlett.8b00308 BindingDB Entry DOI: 10.7270/Q2W0997Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eosinophil peroxidase (Human) | BDBM50507390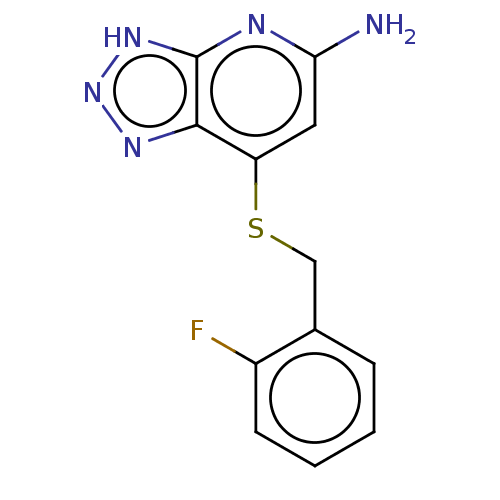 (CHEMBL4482878 | US10981879, Example 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human EPX bromination activity assessed as reduction in H2O2 catalyzed 3-bromo tyrosine formation from tyrosine and potassium bromide p... | ACS Med Chem Lett 9: 1175-1180 (2018) Article DOI: 10.1021/acsmedchemlett.8b00308 BindingDB Entry DOI: 10.7270/Q2W0997Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eosinophil peroxidase (Human) | BDBM357629 (7-benzyl-3H-[1,2,3]triazolo[4,5-b]pyridin-5-amine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human EPX bromination activity using tyrosine as substrate by measuring 3-bromo tyrosine formation incubated for 10 mins | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eosinophil peroxidase (Human) | BDBM434842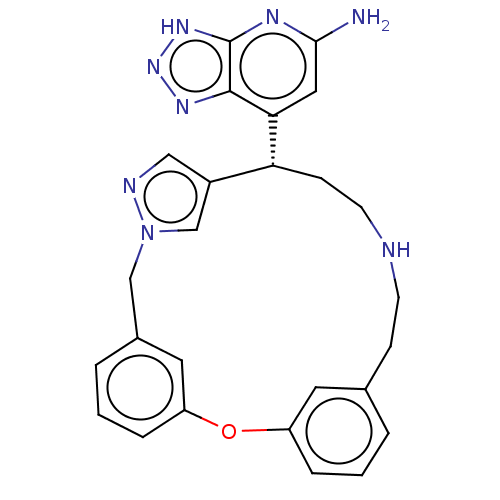 (7-{18-oxa-3,4,10-triazatetracyclo[17.3.1.13,6.113,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi... | US Patent US10577383 (2020) BindingDB Entry DOI: 10.7270/Q2474D8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eosinophil peroxidase (Human) | BDBM434844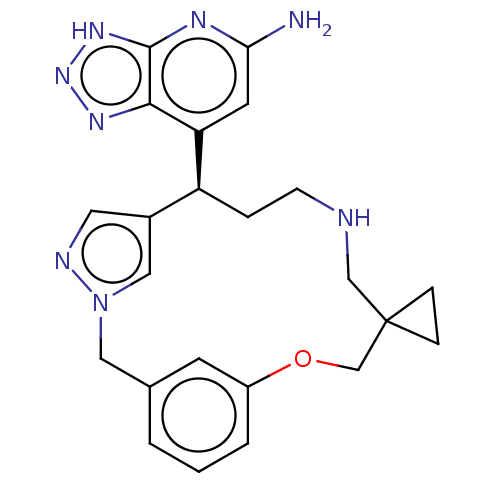 (7-{14′-Oxa-3′,4′,10′-triaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi... | US Patent US10577383 (2020) BindingDB Entry DOI: 10.7270/Q2474D8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eosinophil peroxidase (Human) | BDBM50554034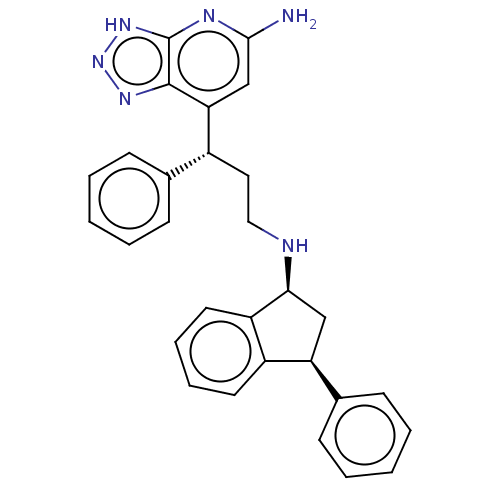 (CHEMBL4747269) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human EPX bromination activity using tyrosine as substrate by measuring 3-bromo tyrosine formation incubated for 10 mins | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eosinophil peroxidase (Human) | BDBM434841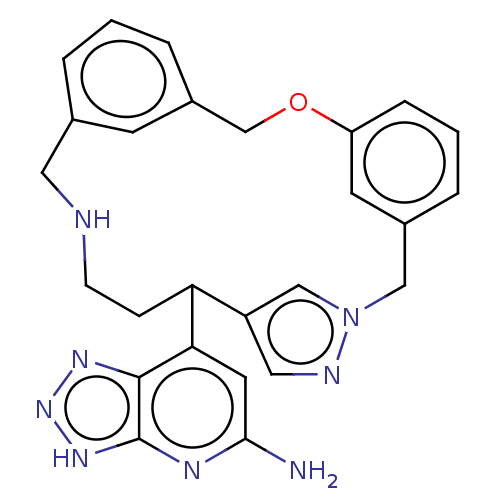 (7-{18-oxa-3,4,10-triazatetracyclo[17.3.1.13,6.112,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi... | US Patent US10577383 (2020) BindingDB Entry DOI: 10.7270/Q2474D8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eosinophil peroxidase (Human) | BDBM434843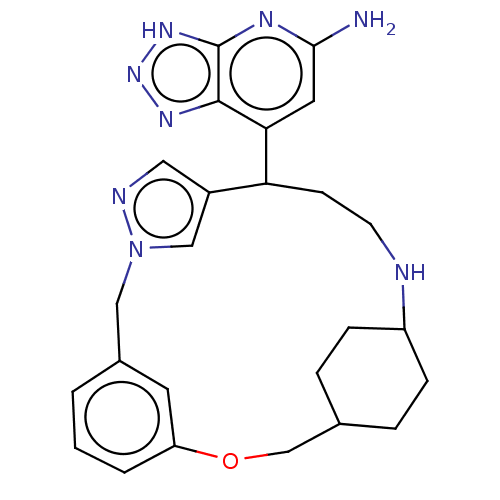 (7-{3-Oxa-10,11,17-triazatetracyclo[16.2.2.14,8.110...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi... | US Patent US10577383 (2020) BindingDB Entry DOI: 10.7270/Q2474D8B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eosinophil peroxidase (Human) | BDBM50554035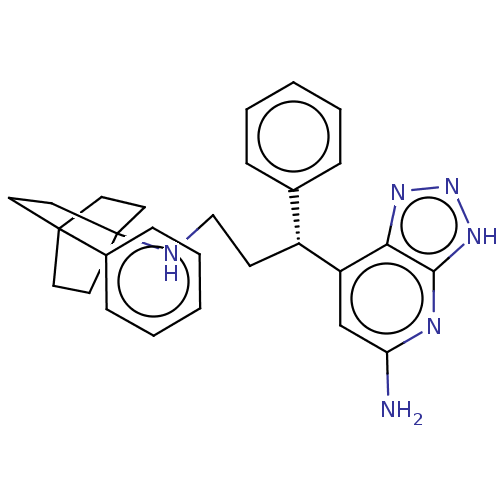 (CHEMBL4790231) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human EPX bromination activity using tyrosine as substrate by measuring 3-bromo tyrosine formation incubated for 10 mins | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||