

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072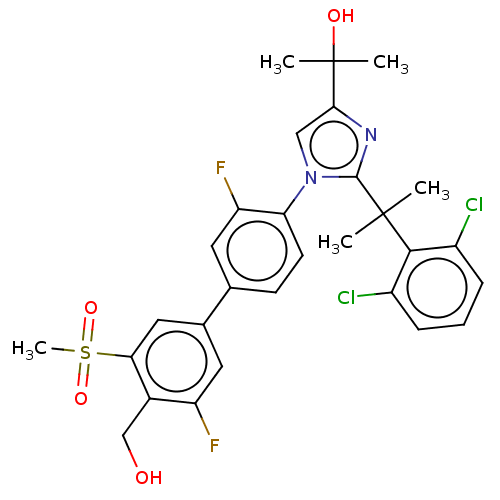 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167698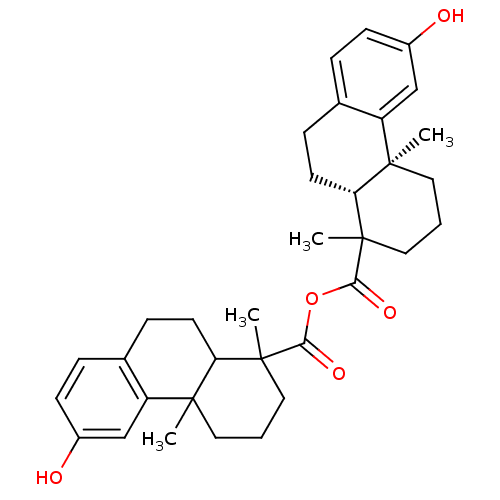 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-beta in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177010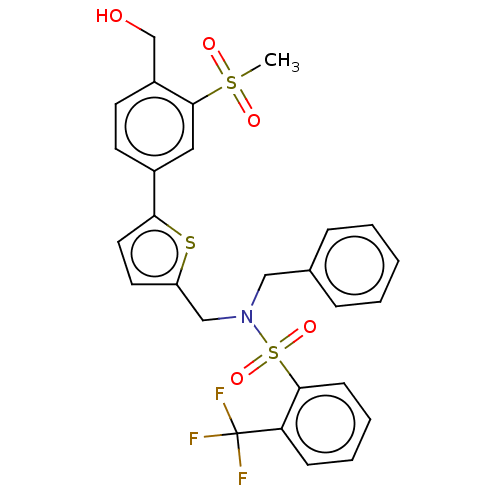 (CHEMBL3814006) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding to human LXRbeta | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204073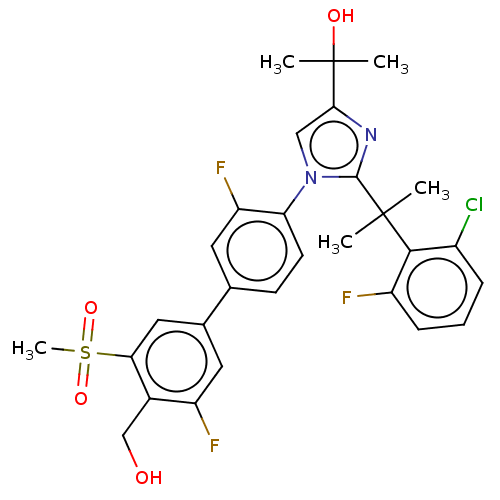 (CHEMBL3917300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415821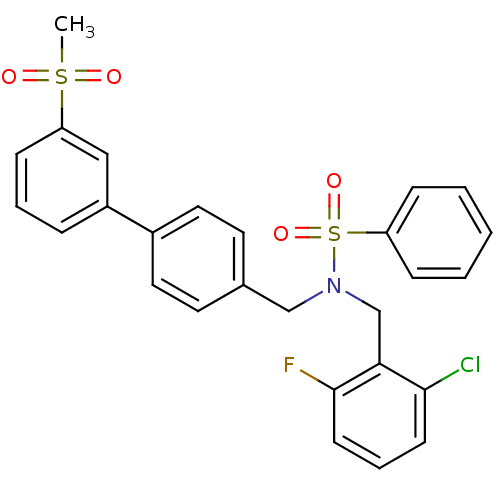 (CHEMBL1093554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204074 (CHEMBL3910597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in recombinant human LXRbeta ligand binding domain in homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204082 (CHEMBL3945820) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204078 (CHEMBL3926292) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50034791 (CHEMBL3360960) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Transactivation of LXRbeta (unknown origin) expressed in CV1 cells by luciferase reporter gene assay | Bioorg Med Chem Lett 25: 372-7 (2014) Article DOI: 10.1016/j.bmcl.2014.11.029 BindingDB Entry DOI: 10.7270/Q2154JN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRbeta receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167698 (13-hydroxy-2,6-dimethyl-(2S,7R)-tricyclo[8.4.0.02,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration for cofactor association with recombinant liver X receptor-beta | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167697 ((4aR,9S)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9,10,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-beta in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50241903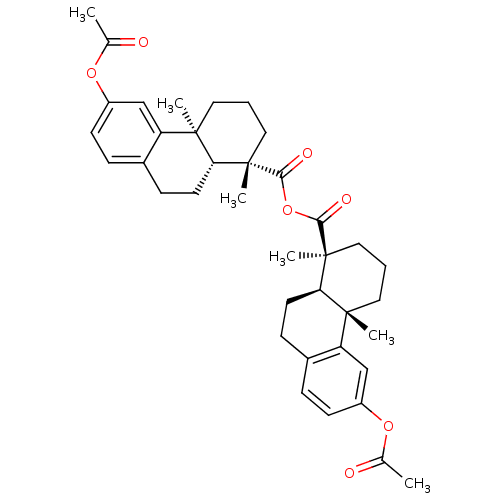 (CHEMBL506838 | acetyl Podocarpic acid anhydride) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | <3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant LXRbeta expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRbeta ligand ... | J Nat Prod 68: 1247-52 (2005) Article DOI: 10.1021/np050182g BindingDB Entry DOI: 10.7270/Q2GH9HQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204079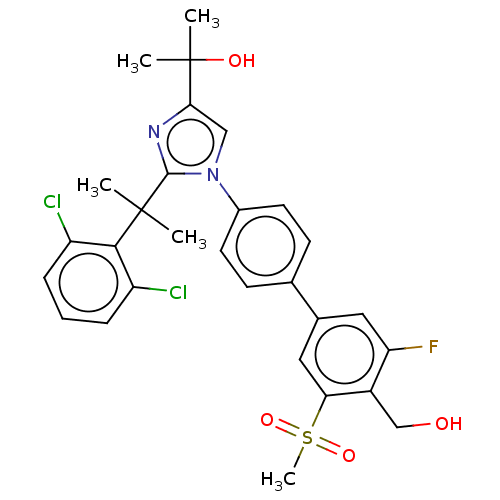 (CHEMBL3935187) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415819 (CHEMBL1091976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50415825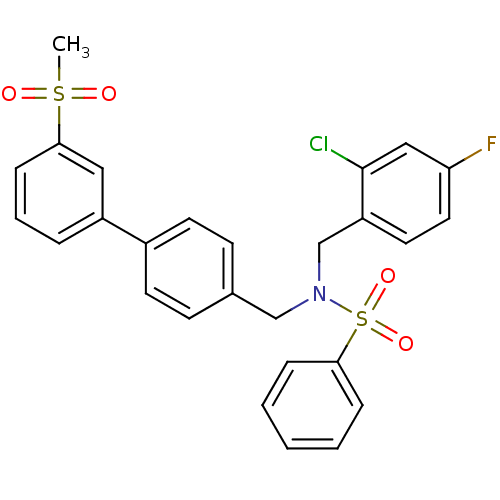 (CHEMBL1091034) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain-mediated transcriptional activity in african green monkey CV1 cells co-transfected with Gal4-SRC1 b... | J Med Chem 53: 3412-6 (2010) Article DOI: 10.1021/jm901797p BindingDB Entry DOI: 10.7270/Q2NP25P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177011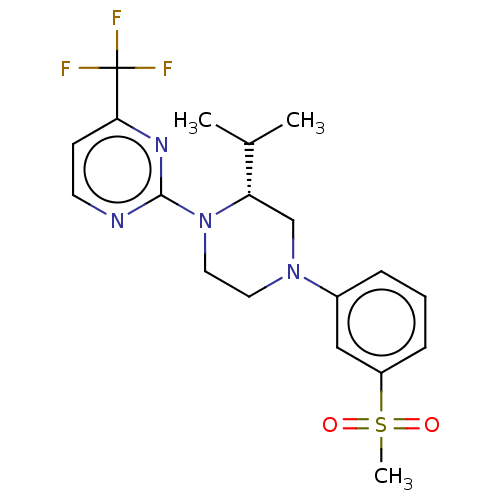 (CHEMBL3815014 | US10144715, Compound 7-13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description This assay is based on the ability of the LXR-LBDs (LXRα and LXRβ) to recruit and interact with a co-activator peptide. This assay was run ... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM27174 (1H-indol-1-yl tertiary amine, 18 | 2-{4-[3-({[2-ch...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4 | n/a | n/a | 7.5 | 22 |
GSK | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | Bioorg Med Chem Lett 19: 1097-100 (2009) Article DOI: 10.1016/j.bmcl.2009.01.004 BindingDB Entry DOI: 10.7270/Q28W3BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304359 ((R)-2-isopropyl-4-(3-(methylsulfonyl)phenyl)-1-(5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description This assay is based on the ability of the LXR-LBDs (LXRα and LXRβ) to recruit and interact with a co-activator peptide. This assay was run ... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at LXRbeta by TR-FRET assay | J Med Chem 54: 788-808 (2012) Article DOI: 10.1021/jm101063h BindingDB Entry DOI: 10.7270/Q208668Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204074 (CHEMBL3910597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human whole blood assessed as ABCA1 gene induction by measuring ABCA1 mRNA level after 4 hrs by SYBR-Green dye-based ... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204075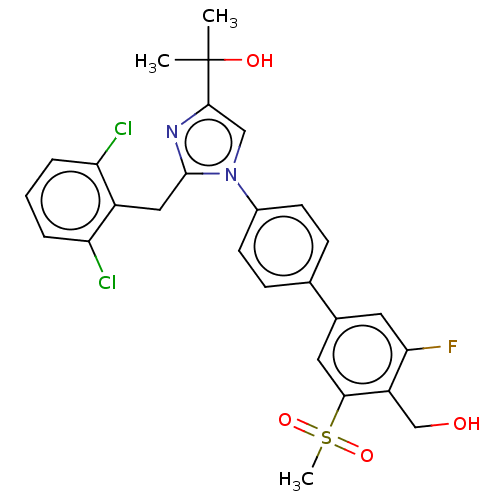 (CHEMBL3980683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding to human LXRbeta | J Med Chem 59: 3264-71 (2016) Article DOI: 10.1021/acs.jmedchem.5b02029 BindingDB Entry DOI: 10.7270/Q2XP76V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20136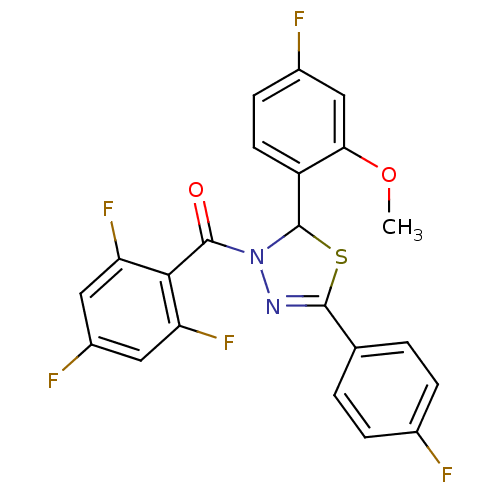 (2-(4-fluoro-2-methoxyphenyl)-5-(4-fluorophenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | 7.2 | 37 |
GNF | Assay Description FRET-based co-activator recruitment assay measures agonist activities in a 384-well plate format. Reaction mixture containing His-tagged hLXR, biotin... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50177012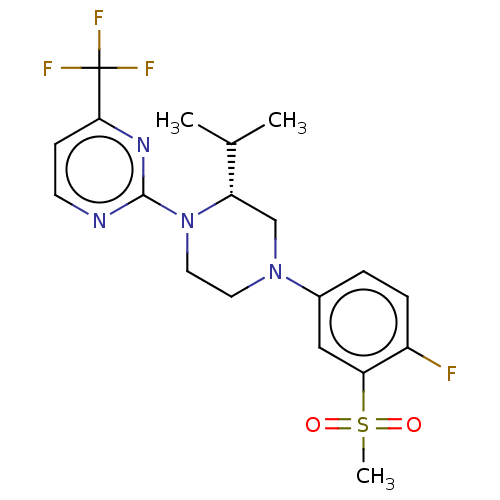 (CHEMBL3814153 | US10144715, Compound 7-32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description This assay is based on the ability of the LXR-LBDs (LXRα and LXRβ) to recruit and interact with a co-activator peptide. This assay was run ... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172624 (CHEMBL3809494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
WuXi AppTec Company, Ltd. Curated by ChEMBL | Assay Description Agonist activity at LXRbeta (unknown origin) expressed in human H4 cells co-expressing ABCA1 promoter measured after 48 hrs by cell-based transactiva... | J Med Chem 59: 3489-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00176 BindingDB Entry DOI: 10.7270/Q2KP843T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM27173 (1H-indol-1-yl tertiary amine, 17 | {[2-chloro-3-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | 7.5 | 22 |
GSK | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | Bioorg Med Chem Lett 19: 1097-100 (2009) Article DOI: 10.1016/j.bmcl.2009.01.004 BindingDB Entry DOI: 10.7270/Q28W3BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50179485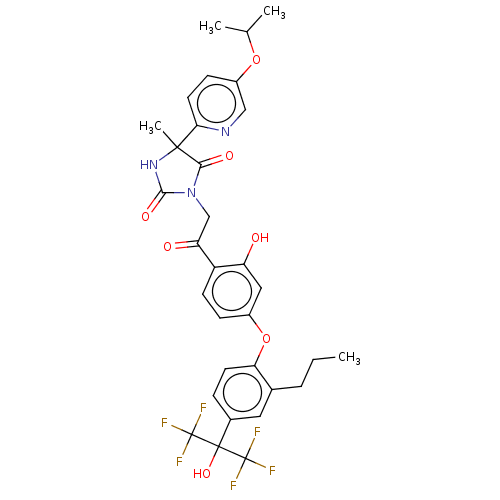 (CHEMBL3814799) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Kowa Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at GAL4 fused LXRbeta-LBD (unknown origin) transfected in CHOK1 cells after 24 hrs by luciferase reporter gene assay | Bioorg Med Chem 24: 3436-46 (2016) Article DOI: 10.1016/j.bmc.2016.05.048 BindingDB Entry DOI: 10.7270/Q2DJ5HJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204071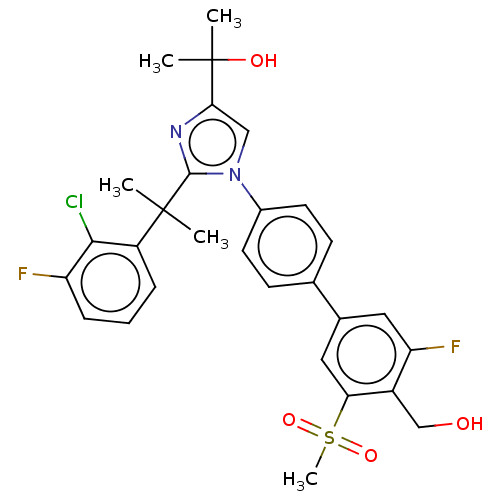 (CHEMBL3953927) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
AstraZeneca R&D Södertälje Curated by ChEMBL | Assay Description Agonist activity at LXRbeta ligand binding domain (unknown origin) by FRET based SRC1 recruitment assay | Bioorg Med Chem Lett 19: 2009-12 (2009) Article DOI: 10.1016/j.bmcl.2009.02.039 BindingDB Entry DOI: 10.7270/Q20C4VNS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204077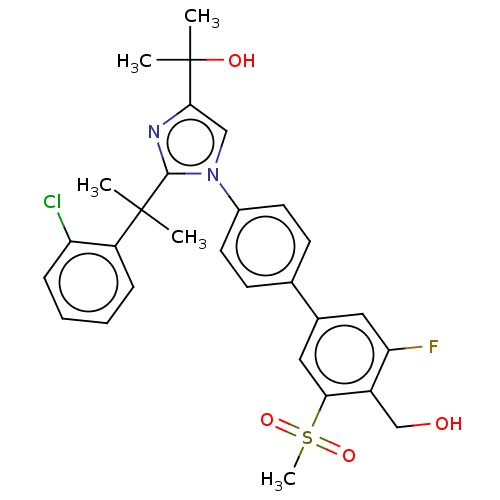 (CHEMBL3944154) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50172624 (CHEMBL3809494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
WuXi AppTec Company, Ltd. Curated by ChEMBL | Assay Description Agonist activity at GST-tagged LXRbeta-LBD (unknown origin) by LanthaScreen TR-FRET liver X receptor coactivator assay | J Med Chem 59: 3489-98 (2016) Article DOI: 10.1021/acs.jmedchem.6b00176 BindingDB Entry DOI: 10.7270/Q2KP843T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human whole blood assessed as ABCA1 gene induction by measuring ABCA1 mRNA level after 4 hrs by SYBR-Green dye-based ... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM304387 (US10144715, Compound 9-2 | methyl (R)-2-(4-(4-fluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description This assay is based on the ability of the LXR-LBDs (LXRα and LXRβ) to recruit and interact with a co-activator peptide. This assay was run ... | US Patent US10144715 (2018) BindingDB Entry DOI: 10.7270/Q2PV6NFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204085 (CHEMBL3960606) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19993 (CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human LXR-beta | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b01126 BindingDB Entry DOI: 10.7270/Q2DF6VVN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human whole blood assessed as ABCG1 gene induction by measuring ABCA1 mRNA level after 4 hrs by SYBR-Green dye-based ... | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration in transactivation assay using a chimeric LXR construct in HEK-293 cells for LXRbeta receptor | Bioorg Med Chem Lett 15: 4574-8 (2005) Article DOI: 10.1016/j.bmcl.2005.06.100 BindingDB Entry DOI: 10.7270/Q2154GKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50426729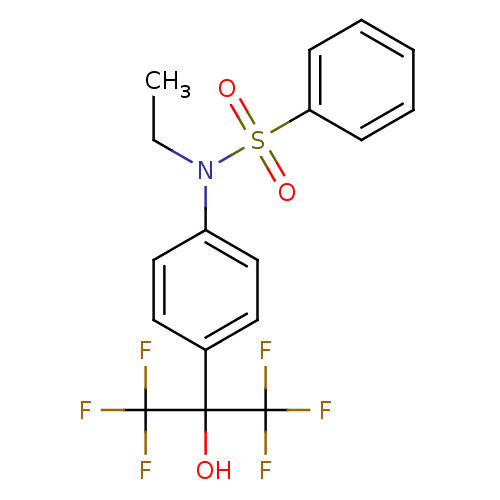 (CHEMBL2326845) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Binding affinity to LXRbeta (unknown origin) | Bioorg Med Chem Lett 23: 579-83 (2012) Article DOI: 10.1016/j.bmcl.2012.11.012 BindingDB Entry DOI: 10.7270/Q2P270GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50167694 (2,6-dimethyl-13-methylcarbonyloxy-(2S)-tricyclo[8....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Effective concentration against liver X receptor-beta in HEK293 cell transactivation assay | Bioorg Med Chem Lett 15: 2824-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.100 BindingDB Entry DOI: 10.7270/Q29G5NM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10543183 (2020) BindingDB Entry DOI: 10.7270/Q2JW8H8C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50300569 ((R)-2-(3-(3-((2-chloro-3-(trifluoromethyl)benzyl)(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta assessed as association of SRC1 to LXRbeta ligand binding domain by FRET based cell-free ligand sensing assay | Bioorg Med Chem Lett 19: 5617-21 (2009) Article DOI: 10.1016/j.bmcl.2009.08.036 BindingDB Entry DOI: 10.7270/Q20865CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204072 (CHEMBL3945199 | US10543183, Compound 12 | US109459...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10945978 (2021) BindingDB Entry DOI: 10.7270/Q2959MPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50204081 (CHEMBL3913162) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at LXR-beta in human HeLa cells assessed as induction of ABCA1 by beta-galactosidase/luciferase reporter gene assay | ACS Med Chem Lett 7: 1207-1212 (2016) Article DOI: 10.1021/acsmedchemlett.6b00234 BindingDB Entry DOI: 10.7270/Q2V98B13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM27273 (3-[4-(1-benzoyl-1H-indol-2-yl)phenyl]propanenitril...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | 7.5 | 22 |
Tanabe Research Laboratories USA | Assay Description Polyhistidine-tagged human LXR ligand-binding domain was mixed with the test compound, biotin-SRC1 peptide, streptavidin-allophycocyanin, and europiu... | Bioorg Med Chem Lett 17: 4442-6 (2007) Article DOI: 10.1016/j.bmcl.2007.06.017 BindingDB Entry DOI: 10.7270/Q2N58JQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM27172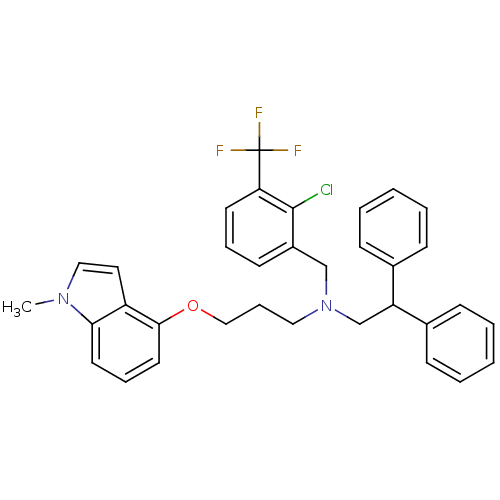 (1H-indol-1-yl tertiary amine, 16 | {[2-chloro-3-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | 7.5 | 22 |
GSK | Assay Description The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding... | Bioorg Med Chem Lett 19: 1097-100 (2009) Article DOI: 10.1016/j.bmcl.2009.01.004 BindingDB Entry DOI: 10.7270/Q28W3BMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1483 total ) | Next | Last >> |