 Found 122 hits of ic50 for UniProtKB: Q92800
Found 122 hits of ic50 for UniProtKB: Q92800 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287072
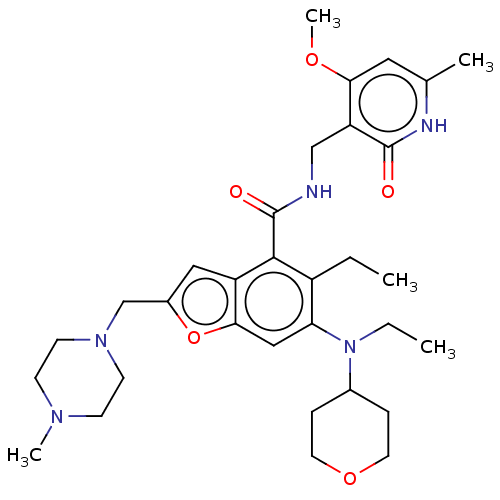
(CHEMBL4159112 | US10759787, Example 12 | US1105981...)Show SMILES CCN(C1CCOCC1)c1cc2oc(CN3CCN(C)CC3)cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1CC Show InChI InChI=1S/C32H45N5O5/c1-6-24-27(37(7-2)22-8-14-41-15-9-22)18-29-25(17-23(42-29)20-36-12-10-35(4)11-13-36)30(24)32(39)33-19-26-28(40-5)16-21(3)34-31(26)38/h16-18,22H,6-15,19-20H2,1-5H3,(H,33,39)(H,34,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287050
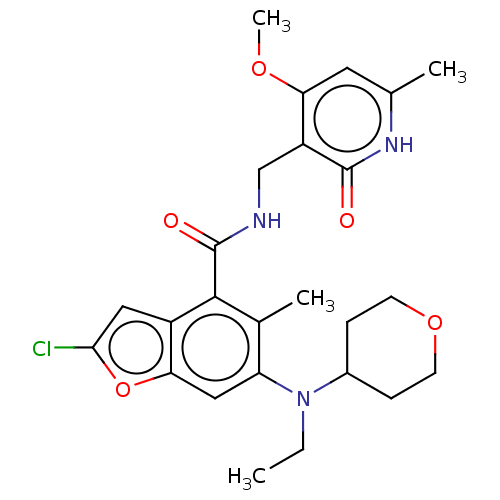
(CHEMBL4161265)Show SMILES CCN(C1CCOCC1)c1cc2oc(Cl)cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C25H30ClN3O5/c1-5-29(16-6-8-33-9-7-16)19-12-21-17(11-22(26)34-21)23(15(19)3)25(31)27-13-18-20(32-4)10-14(2)28-24(18)30/h10-12,16H,5-9,13H2,1-4H3,(H,27,31)(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588052
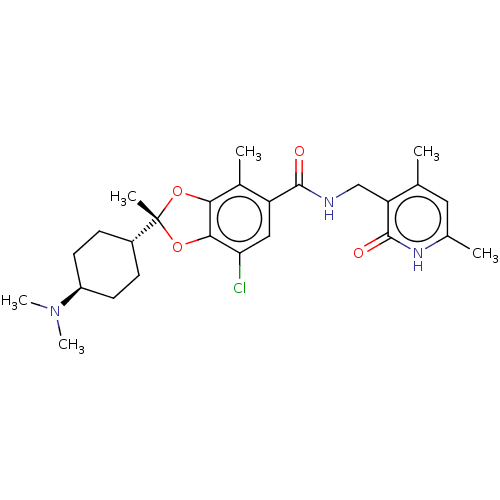
((2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@@]1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(C)cc(C)[nH]c1=O |r,wU:6.6,wD:9.10,3.2,(5.78,-4.8,;4.69,-5.89,;5.09,-7.38,;3.2,-5.49,;2.8,-4.01,;1.32,-3.61,;.23,-4.7,;.63,-6.18,;2.11,-6.58,;-1.26,-4.3,;-2.03,-5.63,;-2.79,-4.14,;-3.11,-2.63,;-1.78,-1.86,;-.63,-2.89,;-1.78,-.32,;-.44,.45,;-3.11,.45,;-4.45,-.32,;-4.45,-1.86,;-5.78,-2.63,;-3.11,1.99,;-4.45,2.76,;-1.78,2.76,;-1.78,4.3,;-.44,5.07,;-.44,6.61,;-1.78,7.38,;.89,7.38,;2.22,6.61,;3.56,7.38,;2.22,5.07,;.89,4.3,;.89,2.76,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50594132
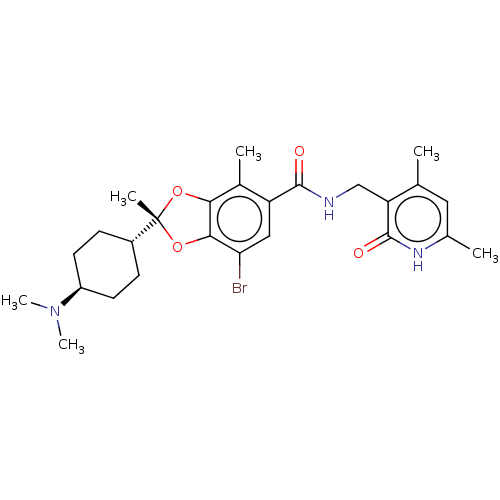
(CHEMBL5197386)Show SMILES [H][C@@]1(CC[C@@H](CC1)N(C)C)[C@@]1(C)Oc2c(O1)c(C)c(cc2Br)C(=O)NCc1c(C)cc(C)[nH]c1=O |r,wU:4.7,1.0,wD:10.11,(3.73,.93,;5.06,.16,;6.39,-.61,;7.73,.16,;7.73,1.7,;6.39,2.47,;5.06,1.7,;9.06,2.47,;9.06,4.01,;10.4,1.7,;3.97,-.93,;5.31,-1.7,;3.07,-2.18,;1.6,-1.7,;1.6,-.16,;3.07,.32,;.27,.61,;.27,2.15,;-1.06,-.16,;-1.06,-1.71,;.28,-2.47,;.28,-4.01,;-2.4,.61,;-2.4,2.15,;-3.73,-.16,;-5.06,.6,;-6.4,-.17,;-6.4,-1.71,;-5.06,-2.48,;-7.73,-2.48,;-9.06,-1.71,;-10.4,-2.48,;-9.06,-.17,;-7.73,.6,;-7.73,2.14,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287052
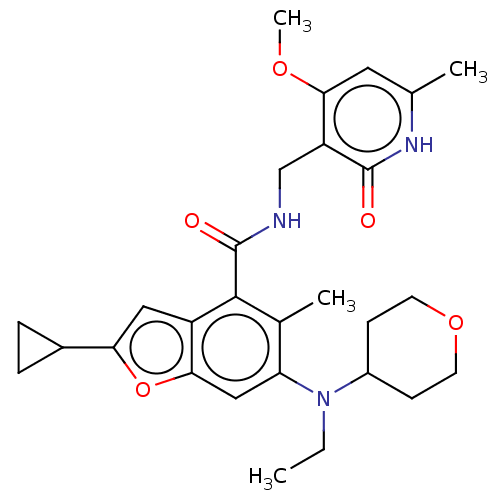
(CHEMBL4165937)Show SMILES CCN(C1CCOCC1)c1cc2oc(cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C)C1CC1 Show InChI InChI=1S/C28H35N3O5/c1-5-31(19-8-10-35-11-9-19)22-14-25-20(13-23(36-25)18-6-7-18)26(17(22)3)28(33)29-15-21-24(34-4)12-16(2)30-27(21)32/h12-14,18-19H,5-11,15H2,1-4H3,(H,29,33)(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287284

(CHEMBL4160111)Show SMILES CCN(C1CCOCC1)c1cc2occc2c(C(=O)NCc2c(CC)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C26H33N3O4/c1-5-18-13-16(3)28-25(30)21(18)15-27-26(31)24-17(4)22(14-23-20(24)9-12-33-23)29(6-2)19-7-10-32-11-8-19/h9,12-14,19H,5-8,10-11,15H2,1-4H3,(H,27,31)(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287068

(CHEMBL4172576 | US10759787, Example 30 | US1105981...)Show SMILES CCN(C1CCOCC1)c1cc2oc(cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C)C#N Show InChI InChI=1S/C26H30N4O5/c1-5-30(17-6-8-34-9-7-17)21-12-23-19(11-18(13-27)35-23)24(16(21)3)26(32)28-14-20-22(33-4)10-15(2)29-25(20)31/h10-12,17H,5-9,14H2,1-4H3,(H,28,32)(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287071

(CHEMBL4164687 | US10759787, Example 29 | US1105981...)Show SMILES CCN(C1CCOCC1)c1cc2oc(cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C)C(F)(F)F Show InChI InChI=1S/C26H30F3N3O5/c1-5-32(16-6-8-36-9-7-16)19-12-21-17(11-22(37-21)26(27,28)29)23(15(19)3)25(34)30-13-18-20(35-4)10-14(2)31-24(18)33/h10-12,16H,5-9,13H2,1-4H3,(H,30,34)(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287125
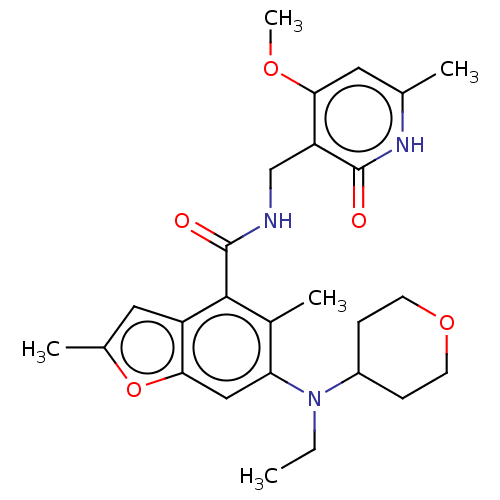
(CHEMBL4170327 | US10759787, Example 19 | US1105981...)Show SMILES CCN(C1CCOCC1)c1cc2oc(C)cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C26H33N3O5/c1-6-29(18-7-9-33-10-8-18)21-13-23-19(12-16(3)34-23)24(17(21)4)26(31)27-14-20-22(32-5)11-15(2)28-25(20)30/h11-13,18H,6-10,14H2,1-5H3,(H,27,31)(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287049

(CHEMBL4162316)Show SMILES CCN(C1CCOCC1)c1cc2occc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1CC Show InChI InChI=1S/C26H33N3O5/c1-5-18-21(29(6-2)17-7-10-33-11-8-17)14-23-19(9-12-34-23)24(18)26(31)27-15-20-22(32-4)13-16(3)28-25(20)30/h9,12-14,17H,5-8,10-11,15H2,1-4H3,(H,27,31)(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287283

(CHEMBL4177454)Show SMILES CCN(C1CCOCC1)c1cc2oc(C)cc2c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C26H33N3O4/c1-6-29(19-7-9-32-10-8-19)22-13-23-20(12-17(4)33-23)24(18(22)5)26(31)27-14-21-15(2)11-16(3)28-25(21)30/h11-13,19H,6-10,14H2,1-5H3,(H,27,31)(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287048
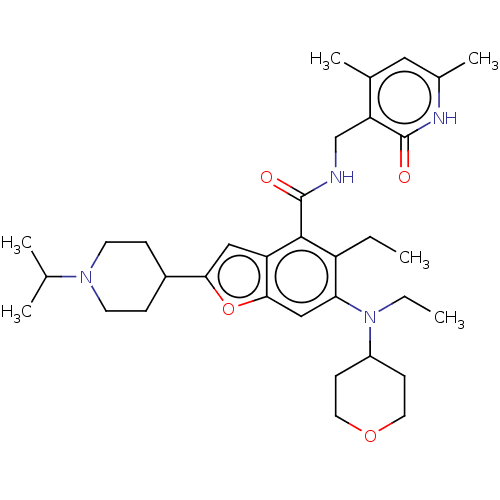
(CHEMBL4162499 | US10759787, Example 9 | US11059811...)Show SMILES CCN(C1CCOCC1)c1cc2oc(cc2c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1CC)C1CCN(CC1)C(C)C Show InChI InChI=1S/C34H48N4O4/c1-7-26-29(38(8-2)25-11-15-41-16-12-25)19-31-27(18-30(42-31)24-9-13-37(14-10-24)21(3)4)32(26)34(40)35-20-28-22(5)17-23(6)36-33(28)39/h17-19,21,24-25H,7-16,20H2,1-6H3,(H,35,40)(H,36,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287054
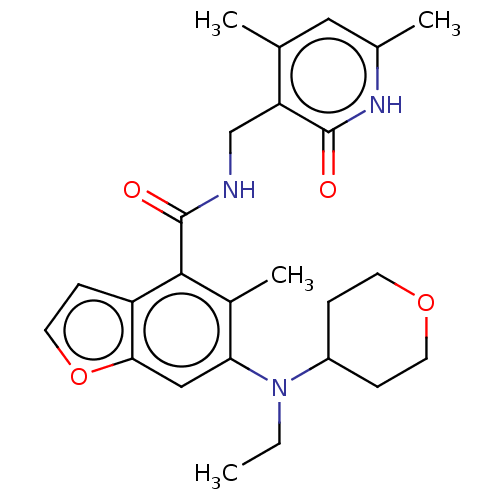
(CHEMBL4174176)Show SMILES CCN(C1CCOCC1)c1cc2occc2c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C25H31N3O4/c1-5-28(18-6-9-31-10-7-18)21-13-22-19(8-11-32-22)23(17(21)4)25(30)26-14-20-15(2)12-16(3)27-24(20)29/h8,11-13,18H,5-7,9-10,14H2,1-4H3,(H,26,30)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287277

(CHEMBL4170753 | US10759787, Example 18 | US1105981...)Show SMILES CCN(C1CCOCC1)c1cc2occc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C25H31N3O5/c1-5-28(17-6-9-32-10-7-17)20-13-22-18(8-11-33-22)23(16(20)3)25(30)26-14-19-21(31-4)12-15(2)27-24(19)29/h8,11-13,17H,5-7,9-10,14H2,1-4H3,(H,26,30)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM172038
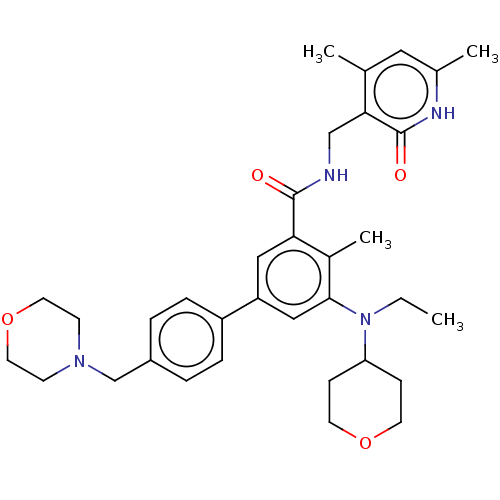
(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287058

(CHEMBL4169191)Show SMILES CCN(C1CCOCC1)c1cc2oc(F)cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C25H30FN3O5/c1-5-29(16-6-8-33-9-7-16)19-12-21-17(11-22(26)34-21)23(15(19)3)25(31)27-13-18-20(32-4)10-14(2)28-24(18)30/h10-12,16H,5-9,13H2,1-4H3,(H,27,31)(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287276

(CHEMBL4162406)Show SMILES CCN(C1CCOCC1)c1cc2occc2c(C(=O)NCc2c(cc(C)[nH]c2=O)C(F)(F)F)c1C Show InChI InChI=1S/C25H28F3N3O4/c1-4-31(16-5-8-34-9-6-16)20-12-21-17(7-10-35-21)22(15(20)3)24(33)29-13-18-19(25(26,27)28)11-14(2)30-23(18)32/h7,10-12,16H,4-6,8-9,13H2,1-3H3,(H,29,33)(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287051

(CHEMBL4169368)Show SMILES CCN(C1CCOCC1)c1cc2oc(CN3CCOCC3)cc2c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1CC Show InChI InChI=1S/C31H42N4O5/c1-5-24-27(35(6-2)22-7-11-38-12-8-22)17-28-25(16-23(40-28)19-34-9-13-39-14-10-34)29(24)31(37)32-18-26-20(3)15-21(4)33-30(26)36/h15-17,22H,5-14,18-19H2,1-4H3,(H,32,37)(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287056

(CHEMBL4169598 | US10759787, Example 20 | US1105981...)Show SMILES CCN(C1CCOCC1)c1cc2occ(C)c2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C26H33N3O5/c1-6-29(18-7-9-33-10-8-18)20-12-22-23(15(2)14-34-22)24(17(20)4)26(31)27-13-19-21(32-5)11-16(3)28-25(19)30/h11-12,14,18H,6-10,13H2,1-5H3,(H,27,31)(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287306

(CHEMBL4167587)Show SMILES CCN(C1CCOCC1)c1cc2sccc2c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C25H31N3O3S/c1-5-28(18-6-9-31-10-7-18)21-13-22-19(8-11-32-22)23(17(21)4)25(30)26-14-20-15(2)12-16(3)27-24(20)29/h8,11-13,18H,5-7,9-10,14H2,1-4H3,(H,26,30)(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287119

(CHEMBL4169762)Show SMILES CCN(C1CCOCC1)c1cc2oc(cc2c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1CC)C1CCN(C)CC1 Show InChI InChI=1S/C32H44N4O4/c1-6-24-27(36(7-2)23-10-14-39-15-11-23)18-29-25(17-28(40-29)22-8-12-35(5)13-9-22)30(24)32(38)33-19-26-20(3)16-21(4)34-31(26)37/h16-18,22-23H,6-15,19H2,1-5H3,(H,33,38)(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287053

(CHEMBL4161444 | US10759787, Example 1 | US11059811...)Show SMILES CCN(C1CCOCC1)c1cc2oc(CN3CCCCC3)cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1CC Show InChI InChI=1S/C32H44N4O5/c1-5-24-27(36(6-2)22-10-14-40-15-11-22)18-29-25(17-23(41-29)20-35-12-8-7-9-13-35)30(24)32(38)33-19-26-28(39-4)16-21(3)34-31(26)37/h16-18,22H,5-15,19-20H2,1-4H3,(H,33,38)(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287047

(CHEMBL4176373)Show SMILES CCN(C1CCOCC1)c1cc2occc2c(C(=O)NCc2ccc(C)[nH]c2=O)c1Cl Show InChI InChI=1S/C23H26ClN3O4/c1-3-27(16-6-9-30-10-7-16)18-12-19-17(8-11-31-19)20(21(18)24)23(29)25-13-15-5-4-14(2)26-22(15)28/h4-5,8,11-12,16H,3,6-7,9-10,13H2,1-2H3,(H,25,29)(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50594116
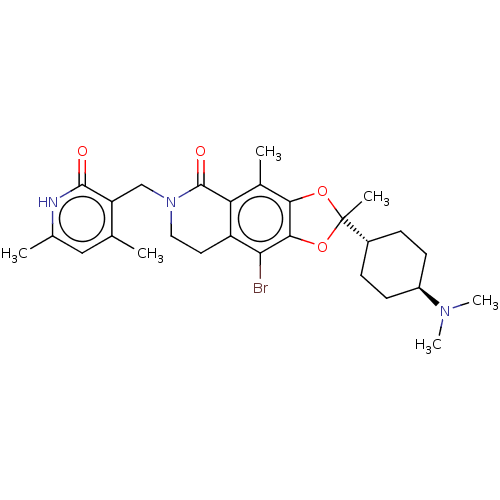
(CHEMBL5185991)Show SMILES [H][C@@]1(CC[C@@H](CC1)N(C)C)C1(C)Oc2c(O1)c(Br)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:4.7,1.0,(4.02,.48,;5.35,-.29,;6.84,-.69,;7.93,.4,;7.53,1.89,;6.04,2.28,;4.95,1.2,;8.62,2.97,;8.22,4.46,;10.11,2.58,;4.26,-1.38,;5.6,-2.15,;3.36,-.14,;1.89,-.61,;1.89,-2.15,;3.36,-2.63,;.57,-2.92,;.57,-4.46,;-.77,-2.16,;-2.11,-2.94,;-3.45,-2.16,;-3.44,-.62,;-4.77,.15,;-6.11,-.62,;-6.1,-2.16,;-4.77,-2.93,;-7.44,-2.93,;-8.77,-2.16,;-10.11,-2.93,;-8.77,-.62,;-7.44,.15,;-7.44,1.69,;-2.11,.15,;-2.11,1.69,;-.77,-.61,;.56,.16,;.56,1.7,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50287307
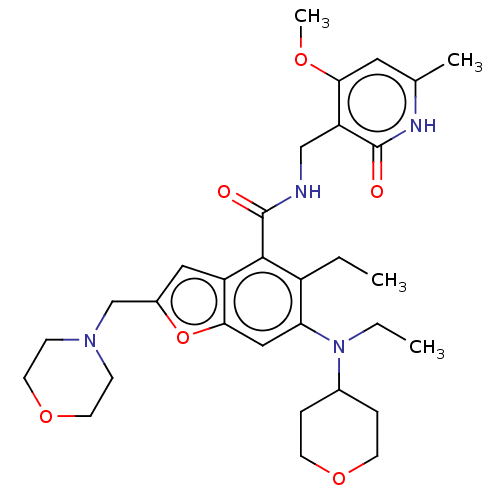
(CHEMBL4176554 | US10759787, Example 6 | US11059811...)Show SMILES CCN(C1CCOCC1)c1cc2oc(CN3CCOCC3)cc2c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c1CC Show InChI InChI=1S/C31H42N4O6/c1-5-23-26(35(6-2)21-7-11-39-12-8-21)17-28-24(16-22(41-28)19-34-9-13-40-14-10-34)29(23)31(37)32-18-25-27(38-4)15-20(3)33-30(25)36/h15-17,21H,5-14,18-19H2,1-4H3,(H,32,37)(H,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588043

((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@]1(C)Oc2c(O1)c(-c1ccco1)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:9.10,3.2,wD:6.5,(10.11,2.58,;8.62,2.97,;8.22,4.46,;7.53,1.89,;7.93,.4,;6.84,-.69,;5.35,-.29,;4.96,1.19,;6.04,2.28,;4.26,-1.38,;5.35,-2.47,;3.36,-2.63,;1.9,-2.15,;1.9,-.61,;3.36,-.14,;.56,.16,;.56,1.7,;1.81,2.6,;1.33,4.07,;-.21,4.07,;-.68,2.6,;-.77,-.61,;-2.11,.16,;-3.44,-.61,;-3.44,-2.15,;-4.77,-2.92,;-6.11,-2.15,;-7.44,-2.92,;-7.44,-4.46,;-8.77,-2.15,;-8.77,-.61,;-10.11,.16,;-7.44,.16,;-6.11,-.61,;-4.77,.16,;-2.11,-2.92,;-2.11,-4.46,;-.77,-2.15,;.56,-2.92,;.56,-4.46,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 8.31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588044

((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@]1(C)Oc2c(O1)c(-c1cccs1)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:9.10,3.2,wD:6.5,(10.11,2.58,;8.62,2.97,;8.22,4.46,;7.53,1.89,;7.93,.4,;6.84,-.69,;5.35,-.29,;4.96,1.19,;6.04,2.28,;4.26,-1.38,;5.35,-2.47,;3.36,-2.63,;1.9,-2.15,;1.9,-.61,;3.36,-.14,;.56,.16,;.56,1.7,;1.81,2.6,;1.33,4.07,;-.21,4.07,;-.68,2.6,;-.77,-.61,;-2.11,.16,;-3.44,-.61,;-3.44,-2.15,;-4.77,-2.92,;-6.11,-2.15,;-7.44,-2.92,;-7.44,-4.46,;-8.77,-2.15,;-8.77,-.61,;-10.11,.16,;-7.44,.16,;-6.11,-.61,;-4.77,.16,;-2.11,-2.92,;-2.11,-4.46,;-.77,-2.15,;.56,-2.92,;.56,-4.46,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588042

(6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-yl)me...)Show SMILES CN(C)C12CCC(CC1)(CC2)C1(C)Oc2c(O1)c(C)c1C(=O)N(Cc3c(C)cc(C)[nH]c3=O)CCc1c2C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50075054
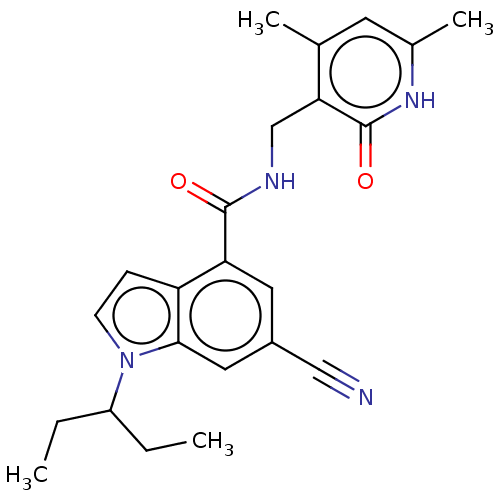
(CHEMBL3414574)Show SMILES CCC(CC)n1ccc2c(cc(cc12)C#N)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C23H26N4O2/c1-5-17(6-2)27-8-7-18-19(10-16(12-24)11-21(18)27)22(28)25-13-20-14(3)9-15(4)26-23(20)29/h7-11,17H,5-6,13H2,1-4H3,(H,25,28)(H,26,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588047

((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@]1(C)Oc2c(O1)c(-c1cccnc1)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:9.10,3.2,wD:6.6,(10.3,1.64,;8.9,2.26,;8.74,3.8,;7.65,1.36,;7.81,-.17,;6.57,-1.08,;5.16,-.45,;5,1.08,;6.24,1.99,;4.07,-1.54,;5.16,-2.63,;3.16,-2.79,;1.7,-2.31,;1.7,-.77,;3.16,-.29,;.37,,;.37,1.54,;-.97,2.31,;-.97,3.85,;.37,4.62,;1.7,3.85,;1.7,2.31,;-.97,-.77,;-2.3,,;-3.63,-.77,;-3.63,-2.31,;-4.97,-3.08,;-6.3,-2.31,;-7.64,-3.08,;-7.64,-4.62,;-8.97,-2.31,;-8.97,-.77,;-10.3,,;-7.64,,;-6.3,-.77,;-4.97,,;-2.3,-3.08,;-2.3,-4.62,;-.97,-2.31,;.37,-3.08,;.37,-4.62,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588041
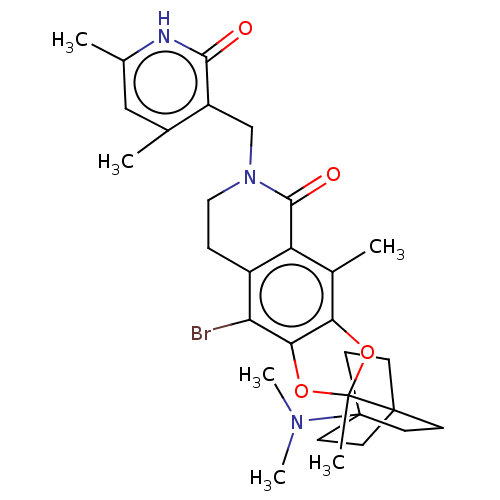
(9-bromo-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin...)Show SMILES CN(C)C12CCC(CC1)(CC2)C1(C)Oc2c(O1)c(Br)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588031

(9-chloro-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridi...)Show SMILES CN(C)C12CCC(CC1)(CC2)C1(C)Oc2c(O1)c(Cl)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588032
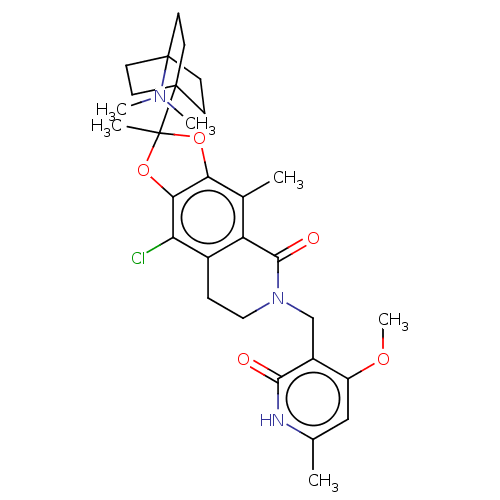
(9-chloro-2-(4- (dimethylamino)bicyclo[2.2.2]octan-...)Show SMILES COc1cc(C)[nH]c(=O)c1CN1CCc2c(Cl)c3OC(C)(Oc3c(C)c2C1=O)C12CCC(CC1)(CC2)N(C)C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588050
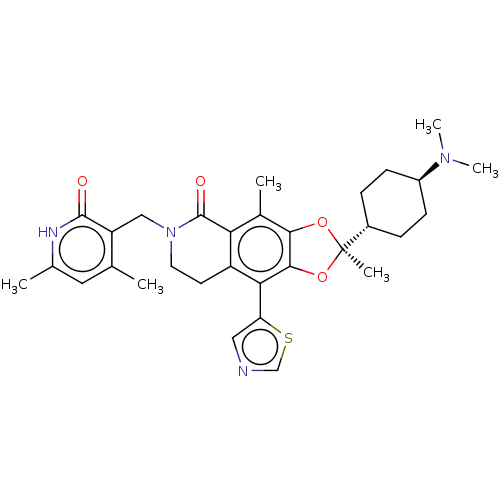
((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@]1(C)Oc2c(O1)c(-c1cncs1)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:9.10,3.2,wD:6.6,(10.3,1.99,;8.9,2.62,;8.74,4.15,;7.65,1.71,;7.81,.18,;6.57,-.72,;5.16,-.1,;5,1.44,;6.24,2.34,;4.07,-1.18,;5.16,-2.27,;3.16,-2.43,;1.7,-1.95,;1.7,-.41,;3.16,.06,;.37,.36,;.37,1.9,;1.61,2.8,;1.14,4.26,;-.4,4.26,;-.88,2.8,;-.97,-.41,;-2.3,.36,;-3.63,-.41,;-3.63,-1.95,;-4.97,-2.72,;-6.3,-1.95,;-7.64,-2.72,;-7.64,-4.26,;-8.97,-1.95,;-8.97,-.41,;-10.3,.36,;-7.64,.36,;-6.3,-.41,;-4.97,.36,;-2.3,-2.72,;-2.3,-4.26,;-.97,-1.95,;.37,-2.72,;.37,-4.26,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM50149895

(CHEMBL3770000)Show SMILES CCN(C1CCOCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C23H30BrN3O3/c1-5-27(18-6-8-30-9-7-18)21-12-17(24)11-19(16(21)4)22(28)25-13-20-14(2)10-15(3)26-23(20)29/h10-12,18H,5-9,13H2,1-4H3,(H,25,28)(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Hengrui Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged EZH2/flag-tagged EED/SUZ12/AEBP2/RBAP48 A677G mutant (2 to end residues) expressed in baculovirus infected ... |
ACS Med Chem Lett 9: 98-102 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00437
BindingDB Entry DOI: 10.7270/Q2ZS3028 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588048
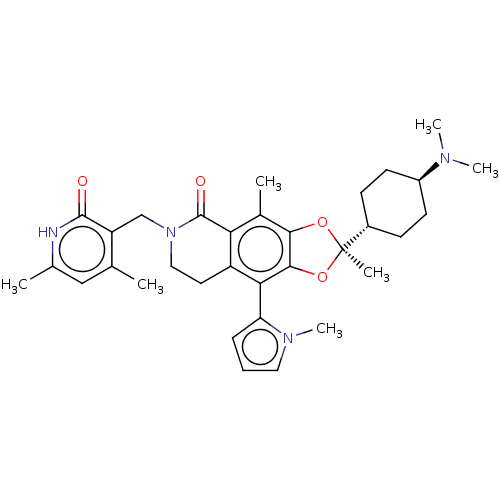
((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@]1(C)Oc2c(O1)c(-c1cccn1C)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:9.10,3.2,wD:6.6,(10.3,1.99,;8.9,2.62,;8.74,4.15,;7.65,1.71,;7.81,.18,;6.57,-.72,;5.16,-.1,;5,1.44,;6.24,2.34,;4.07,-1.18,;5.16,-2.27,;3.16,-2.43,;1.7,-1.95,;1.7,-.41,;3.16,.06,;.37,.36,;.37,1.9,;1.61,2.8,;1.14,4.26,;-.4,4.26,;-.88,2.8,;-2.34,2.32,;-.97,-.41,;-2.3,.36,;-3.63,-.41,;-3.63,-1.95,;-4.97,-2.72,;-6.3,-1.95,;-7.64,-2.72,;-7.64,-4.26,;-8.97,-1.95,;-8.97,-.41,;-10.3,.36,;-7.64,.36,;-6.3,-.41,;-4.97,.36,;-2.3,-2.72,;-2.3,-4.26,;-.97,-1.95,;.37,-2.72,;.37,-4.26,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50590375
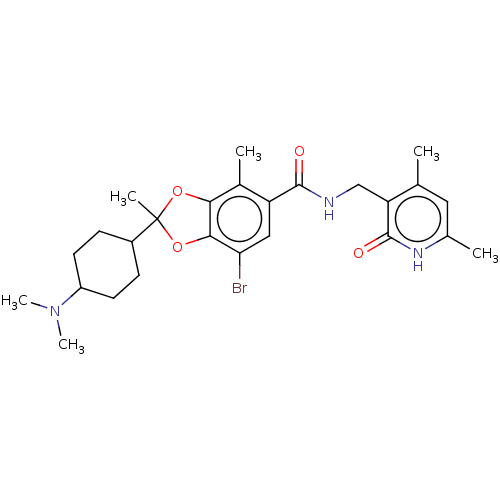
(CHEMBL5187396)Show SMILES CN(C)C1CCC(CC1)C1(C)Oc2c(O1)c(C)c(cc2Br)C(=O)NCc1c(C)cc(C)[nH]c1=O |(2.94,-8.67,;1.46,-8.27,;.37,-9.36,;1.06,-6.79,;2.15,-5.7,;1.75,-4.21,;.26,-3.81,;-.83,-4.9,;-.43,-6.39,;-.14,-2.32,;1.4,-2.32,;-1.67,-2.17,;-1.99,-.66,;-.66,.11,;.49,-.92,;-.66,1.66,;.67,2.43,;-2,2.43,;-3.34,1.66,;-3.34,.11,;-4.67,-.66,;-2,3.97,;-3.33,4.74,;-.66,4.74,;-.66,6.28,;.67,7.05,;.67,8.59,;-.66,9.36,;2,9.36,;3.34,8.59,;4.67,9.36,;3.34,7.05,;2,6.28,;2,4.74,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114419
BindingDB Entry DOI: 10.7270/Q2PR80Z0 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588049
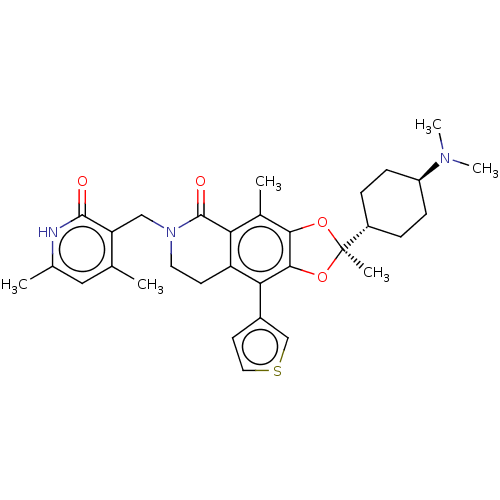
((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@]1(C)Oc2c(O1)c(-c1ccsc1)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:9.10,3.2,wD:6.6,(10.3,1.99,;8.9,2.62,;8.74,4.15,;7.65,1.71,;7.81,.18,;6.57,-.72,;5.16,-.1,;5,1.44,;6.24,2.34,;4.07,-1.18,;5.16,-2.27,;3.16,-2.43,;1.7,-1.95,;1.7,-.41,;3.16,.06,;.37,.36,;.37,1.9,;1.61,2.8,;1.14,4.26,;-.4,4.26,;-.88,2.8,;-.97,-.41,;-2.3,.36,;-3.63,-.41,;-3.63,-1.95,;-4.97,-2.72,;-6.3,-1.95,;-7.64,-2.72,;-7.64,-4.26,;-8.97,-1.95,;-8.97,-.41,;-10.3,.36,;-7.64,.36,;-6.3,-.41,;-4.97,.36,;-2.3,-2.72,;-2.3,-4.26,;-.97,-1.95,;.37,-2.72,;.37,-4.26,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588046

((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@]1(C)Oc2c(O1)c(-c1ccc(C)o1)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:9.10,3.2,wD:6.6,(10.3,1.37,;8.9,2,;8.74,3.53,;7.65,1.09,;7.81,-.44,;6.57,-1.35,;5.16,-.72,;5,.81,;6.24,1.72,;4.07,-1.81,;5.16,-2.9,;3.16,-3.05,;1.7,-2.58,;1.7,-1.04,;3.16,-.56,;.37,-.27,;.37,1.27,;1.61,2.18,;1.14,3.64,;-.4,3.64,;-1.31,4.89,;-.88,2.18,;-.97,-1.04,;-2.3,-.27,;-3.63,-1.04,;-3.63,-2.58,;-4.97,-3.35,;-6.3,-2.58,;-7.64,-3.35,;-7.64,-4.89,;-8.97,-2.58,;-8.97,-1.04,;-10.3,-.27,;-7.64,-.27,;-6.3,-1.04,;-4.97,-.27,;-2.3,-3.35,;-2.3,-4.89,;-.97,-2.58,;.37,-3.35,;.37,-4.89,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50590376

(CHEMBL5193486)Show SMILES CN(C)C1CCC(CC1)C1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(C)cc(C)[nH]c1=O |(2.94,-8.67,;1.46,-8.27,;.37,-9.36,;1.06,-6.79,;2.15,-5.7,;1.75,-4.21,;.26,-3.81,;-.83,-4.9,;-.43,-6.39,;-.14,-2.32,;1.4,-2.32,;-1.67,-2.17,;-1.99,-.66,;-.66,.11,;.49,-.92,;-.66,1.66,;.67,2.43,;-2,2.43,;-3.34,1.66,;-3.34,.11,;-4.67,-.66,;-2,3.97,;-3.33,4.74,;-.66,4.74,;-.66,6.28,;.67,7.05,;.67,8.59,;-.66,9.36,;2,9.36,;3.34,8.59,;4.67,9.36,;3.34,7.05,;2,6.28,;2,4.74,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114419
BindingDB Entry DOI: 10.7270/Q2PR80Z0 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588052

((2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@@]1(C)Oc2c(O1)c(C)c(cc2Cl)C(=O)NCc1c(C)cc(C)[nH]c1=O |r,wU:6.6,wD:9.10,3.2,(5.78,-4.8,;4.69,-5.89,;5.09,-7.38,;3.2,-5.49,;2.8,-4.01,;1.32,-3.61,;.23,-4.7,;.63,-6.18,;2.11,-6.58,;-1.26,-4.3,;-2.03,-5.63,;-2.79,-4.14,;-3.11,-2.63,;-1.78,-1.86,;-.63,-2.89,;-1.78,-.32,;-.44,.45,;-3.11,.45,;-4.45,-.32,;-4.45,-1.86,;-5.78,-2.63,;-3.11,1.99,;-4.45,2.76,;-1.78,2.76,;-1.78,4.3,;-.44,5.07,;-.44,6.61,;-1.78,7.38,;.89,7.38,;2.22,6.61,;3.56,7.38,;2.22,5.07,;.89,4.3,;.89,2.76,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| US Patent
| n/a | n/a | 31.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM588045

((R)-6-((4,6-dimethyl-2-oxo-1,2- dihydropyridin-3-y...)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)[C@]1(C)Oc2c(O1)c(-c1ccncc1)c1CCN(Cc3c(C)cc(C)[nH]c3=O)C(=O)c1c2C |r,wU:9.10,3.2,wD:6.6,(10.3,1.64,;8.9,2.26,;8.74,3.8,;7.65,1.36,;7.81,-.17,;6.57,-1.08,;5.16,-.45,;5,1.08,;6.24,1.99,;4.07,-1.54,;5.16,-2.63,;3.16,-2.79,;1.7,-2.31,;1.7,-.77,;3.16,-.29,;.37,,;.37,1.54,;1.7,2.31,;1.7,3.85,;.37,4.62,;-.97,3.85,;-.97,2.31,;-.97,-.77,;-2.3,,;-3.63,-.77,;-3.63,-2.31,;-4.97,-3.08,;-6.3,-2.31,;-7.64,-3.08,;-7.64,-4.62,;-8.97,-2.31,;-8.97,-.77,;-10.3,,;-7.64,,;-6.3,-.77,;-4.97,,;-2.3,-3.08,;-2.3,-4.62,;-.97,-2.31,;.37,-3.08,;.37,-4.62,)| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| UniChem
| US Patent
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The inhibitory activity of the synthesized compounds above against EZH1 or EZH2 methyltransferase was measured. The experiments were consigned to Rea... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2PV6Q7J |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50594130

(CHEMBL5196227)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2N(NCc12)C(C)C)-c1ccc(nc1)N1CCN(CC1)C(C)C | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50075051
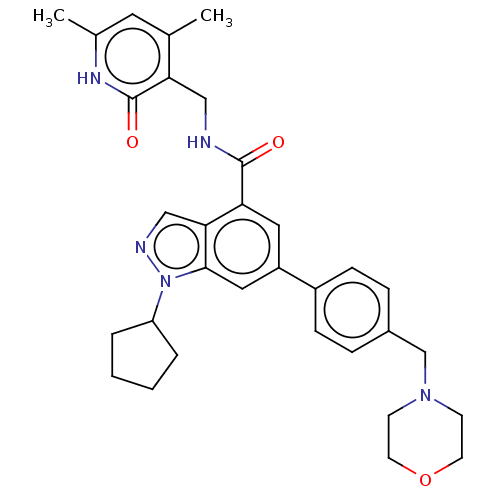
(CHEMBL3360855 | EPZ005687 | US10273223, Compound C...)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(cc3n(ncc23)C2CCCC2)-c2ccc(CN3CCOCC3)cc2)c(=O)[nH]1 Show InChI InChI=1S/C32H37N5O3/c1-21-15-22(2)35-32(39)28(21)18-33-31(38)27-16-25(17-30-29(27)19-34-37(30)26-5-3-4-6-26)24-9-7-23(8-10-24)20-36-11-13-40-14-12-36/h7-10,15-17,19,26H,3-6,11-14,18,20H2,1-2H3,(H,33,38)(H,35,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH1 in PRC2 complex (unknown origin) using biotinylated peptide as substrate in presence of S-adenosylmethionine |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01344
BindingDB Entry DOI: 10.7270/Q23R0XMC |
More data for this
Ligand-Target Pair | |
Histone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2
(Homo sapiens (Human)) | BDBM225230

(EED226 | US11013745, Compound EED226)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(NCc2ccco2)n2cnnc12 Show InChI InChI=1S/C17H15N5O3S/c1-26(23,24)14-6-4-12(5-7-14)15-10-19-17(22-11-20-21-16(15)22)18-9-13-3-2-8-25-13/h2-8,10-11H,9H2,1H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
| Assay Description
All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383]. |
Nat Chem Biol 13: 381-388 (2017)
Article DOI: 10.1038/nchembio.2304
BindingDB Entry DOI: 10.7270/Q2S75F64 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50541920

(Cpi-1205 | Lirametostat)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC(F)(F)F)CC2)c2ccccc12 Show InChI InChI=1S/C27H33F3N4O3/c1-16-13-23(37-4)21(25(35)32-16)14-31-26(36)24-18(3)34(22-8-6-5-7-20(22)24)17(2)19-9-11-33(12-10-19)15-27(28,29)30/h5-8,13,17,19H,9-12,14-15H2,1-4H3,(H,31,36)(H,32,35)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH1 (unknown origin) |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50536604
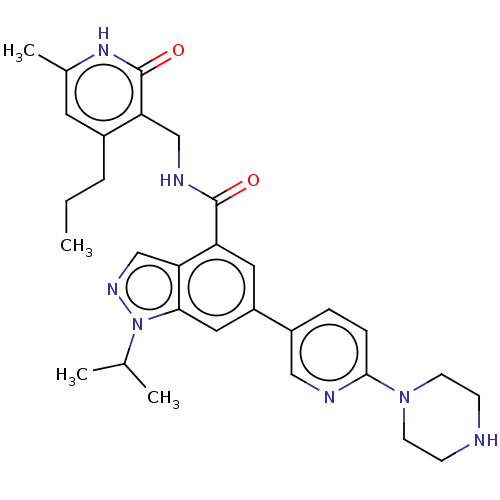
(CHEMBL4594174)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccc(nc1)N1CCNCC1 Show InChI InChI=1S/C30H37N7O2/c1-5-6-21-13-20(4)35-30(39)25(21)17-33-29(38)24-14-23(15-27-26(24)18-34-37(27)19(2)3)22-7-8-28(32-16-22)36-11-9-31-10-12-36/h7-8,13-16,18-19,31H,5-6,9-12,17H2,1-4H3,(H,33,38)(H,35,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... |
J Med Chem 59: 7617-33 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00855
BindingDB Entry DOI: 10.7270/Q2JW8JC4 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50075071

(CHEMBL3414619)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccc(nc1)N1CCN(CC1)C(C)C Show InChI InChI=1S/C33H43N7O2/c1-7-8-24-15-23(6)37-33(42)28(24)19-35-32(41)27-16-26(17-30-29(27)20-36-40(30)22(4)5)25-9-10-31(34-18-25)39-13-11-38(12-14-39)21(2)3/h9-10,15-18,20-22H,7-8,11-14,19H2,1-6H3,(H,35,41)(H,37,42) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of EZH1 histone methyltransferase activity of EZH1/EED/SUZ12/RBBP4/AEBP2 protein complex (unknown origin) using histone H3 peptide as subs... |
J Med Chem 59: 7617-33 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00855
BindingDB Entry DOI: 10.7270/Q2JW8JC4 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
(Homo sapiens (Human)) | BDBM50594155
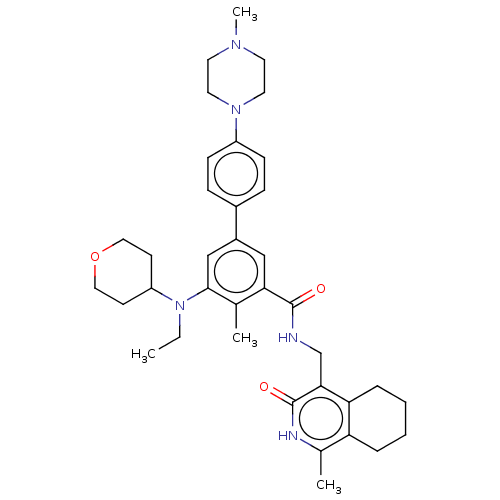
(CHEMBL5203667)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c3CCCCc3c(C)[nH]c2=O)c1C)-c1ccc(cc1)N1CCN(C)CC1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH1
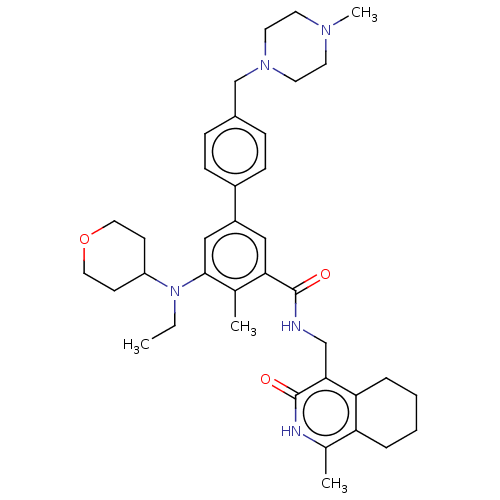
(Homo sapiens (Human)) | BDBM50544756
(CHEMBL4637350)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c3CCCCc3c(C)[nH]c2=O)c1C)-c1ccc(CN2CCN(C)CC2)cc1 Show InChI InChI=1S/C38H51N5O3/c1-5-43(31-14-20-46-21-15-31)36-23-30(29-12-10-28(11-13-29)25-42-18-16-41(4)17-19-42)22-34(26(36)2)37(44)39-24-35-33-9-7-6-8-32(33)27(3)40-38(35)45/h10-13,22-23,31H,5-9,14-21,24-25H2,1-4H3,(H,39,44)(H,40,45) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00047
BindingDB Entry DOI: 10.7270/Q27D3059 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data