

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM139540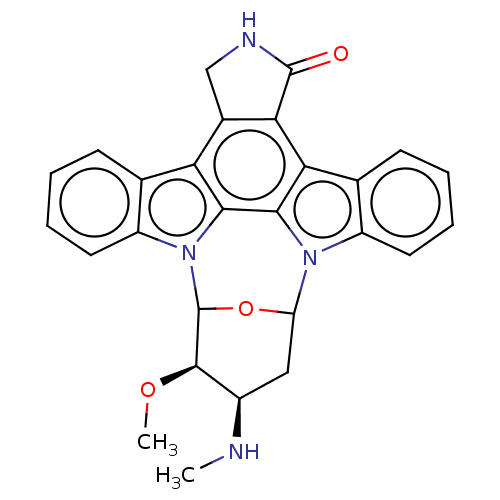 (US10189849, staurosporine | US10307427, Staurospor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM50377170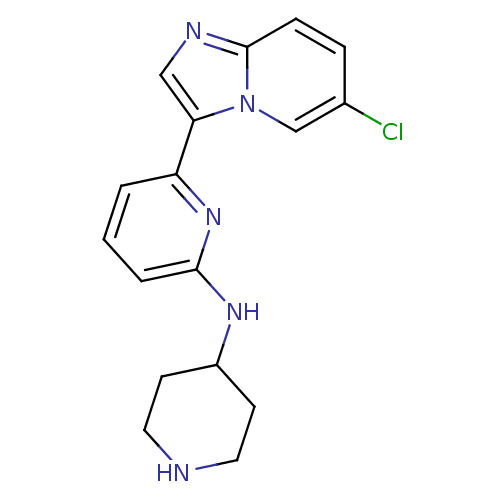 (CHEMBL256570 | US11254667, Compound I-2 | US115422...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM50377170 (CHEMBL256570 | US11254667, Compound I-2 | US115422...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.236 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.236 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50377170 (CHEMBL256570 | US11254667, Compound I-2 | US115422...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.356 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM139540 (US10189849, staurosporine | US10307427, Staurospor...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.356 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50377170 (CHEMBL256570 | US11254667, Compound I-2 | US115422...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.356 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50377170 (CHEMBL256570 | US11254667, Compound I-2 | US115422...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538619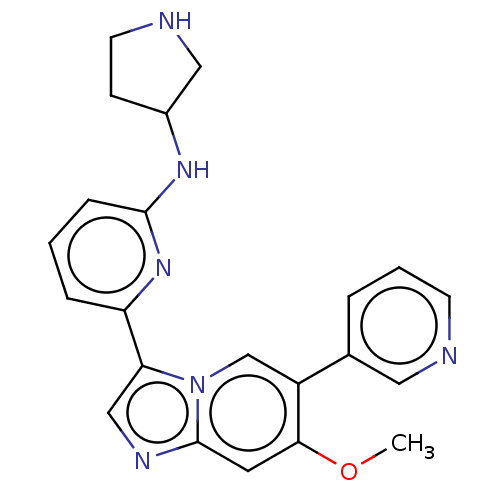 (NCGC 00371479 | US11254667, Compound I-22 | US1154...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538621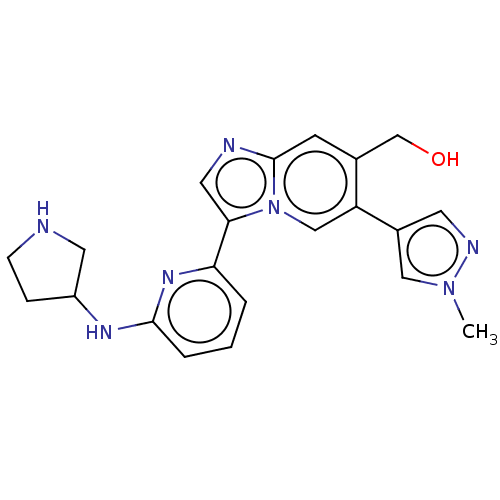 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM538619 (NCGC 00371479 | US11254667, Compound I-22 | US1154...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538619 (NCGC 00371479 | US11254667, Compound I-22 | US1154...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM538619 (NCGC 00371479 | US11254667, Compound I-22 | US1154...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM139540 (US10189849, staurosporine | US10307427, Staurospor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM538618 (NCGC 00262327 | US11254667, Compound I-20 | US1154...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM139540 (US10189849, staurosporine | US10307427, Staurospor...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM538618 (NCGC 00262327 | US11254667, Compound I-20 | US1154...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM538618 (NCGC 00262327 | US11254667, Compound I-20 | US1154...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM139540 (US10189849, staurosporine | US10307427, Staurospor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538619 (NCGC 00371479 | US11254667, Compound I-22 | US1154...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538618 (NCGC 00262327 | US11254667, Compound I-20 | US1154...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538658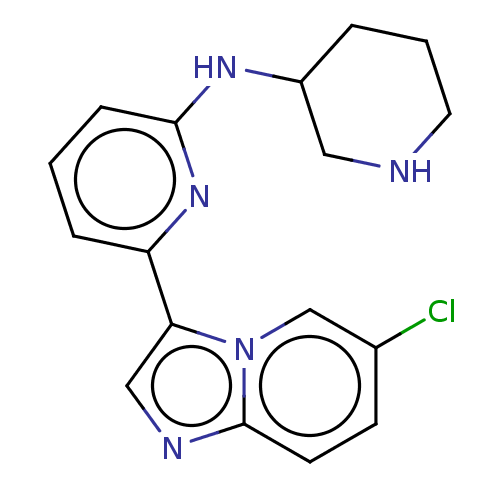 (NCGC 00249373 | US11254667, Compound I-17 | US1154...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM139540 (US10189849, staurosporine | US10307427, Staurospor...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM538619 (NCGC 00371479 | US11254667, Compound I-22 | US1154...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM538618 (NCGC 00262327 | US11254667, Compound I-20 | US1154...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM538619 (NCGC 00371479 | US11254667, Compound I-22 | US1154...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM139540 (US10189849, staurosporine | US10307427, Staurospor...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538658 (NCGC 00249373 | US11254667, Compound I-17 | US1154...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538618 (NCGC 00262327 | US11254667, Compound I-20 | US1154...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538619 (NCGC 00371479 | US11254667, Compound I-22 | US1154...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM538621 (NCGC 00371481 | US11254667, Compound I-24 | US1154...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM139540 (US10189849, staurosporine | US10307427, Staurospor...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM538658 (NCGC 00249373 | US11254667, Compound I-17 | US1154...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 [D835Y] (Homo sapiens (Human)) | BDBM538618 (NCGC 00262327 | US11254667, Compound I-20 | US1154...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.508 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 8-10: Compounds were tested in 10-dose IC50 mode with 3-fold serial dilution starting at 10 uM, and are relative to DMSO, the negative control.... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM139540 (US10189849, staurosporine | US10307427, Staurospor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.623 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 7 For most assays, kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phas... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.623 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 7 For most assays, kinase-tagged T7 phage strains were prepared in an E. coli host derived from the BL21 strain. E. coli were grown to log-phas... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-H/Cyclin-dependent kinase 7 (Homo sapiens (Human)) | BDBM50300690 (1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 0.623 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Table 3 and 4: The HotSpot kinase profiling and screening assays were carried out using the method of Anastassiadis et al., Nat. Biotechnol. (2011) V... | Citation and Details BindingDB Entry DOI: 10.7270/Q2154MW8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 293 total ) | Next | Last >> |