

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001119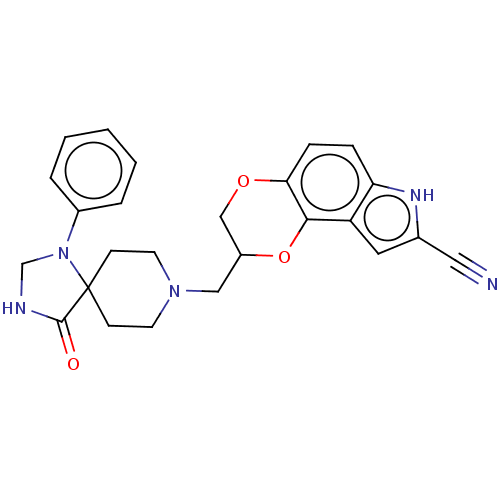 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards opioid receptor from rat whole brain using [3H]etorpine (33.2 Ci/mmol,0.2nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001117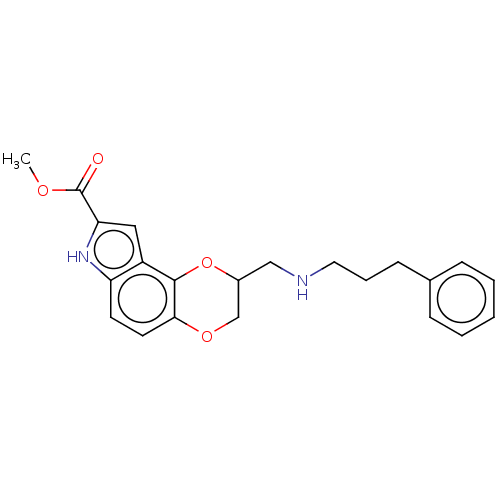 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001118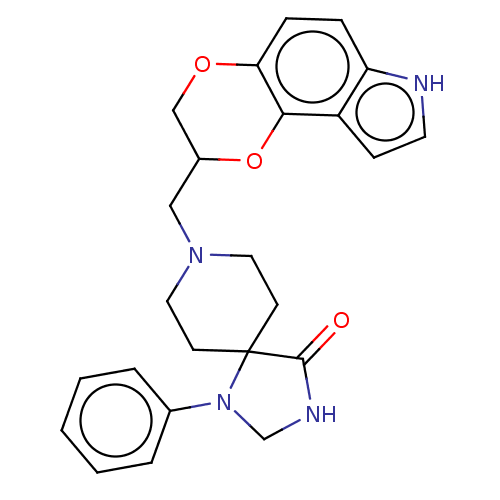 (8-(7,8-Dihydro-3H-6,9-dioxa-3-aza-cyclopenta[a]nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/m... | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001113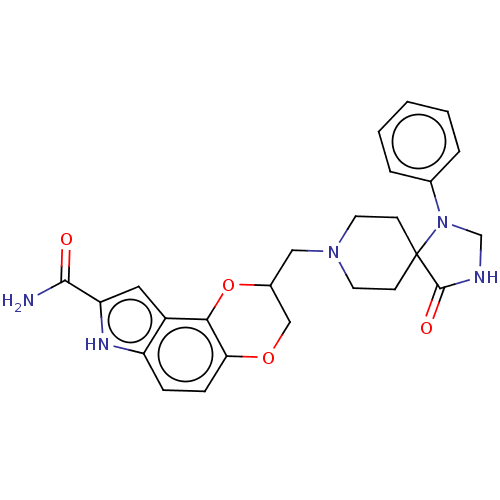 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001118 (8-(7,8-Dihydro-3H-6,9-dioxa-3-aza-cyclopenta[a]nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001119 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001113 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards opioid receptor from rat whole brain using [3H]etorpine (33.2 Ci/mmol,0.2nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001116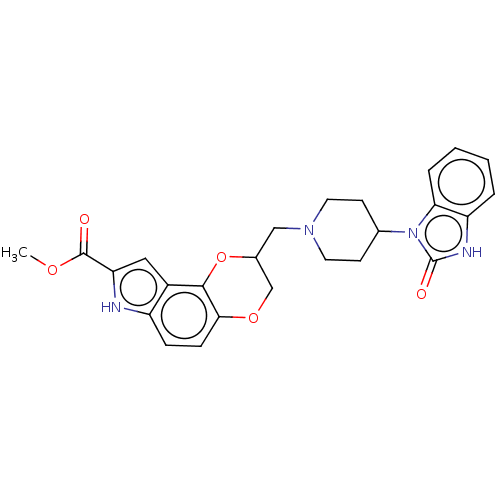 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001117 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001112 (8-(2-Hydroxymethyl-7,8-dihydro-3H-6,9-dioxa-3-aza-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001114 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001110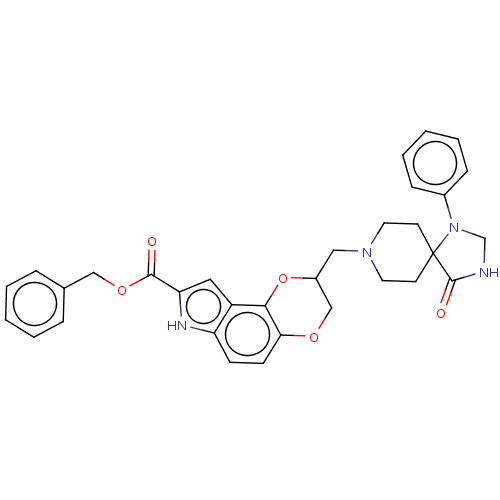 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001111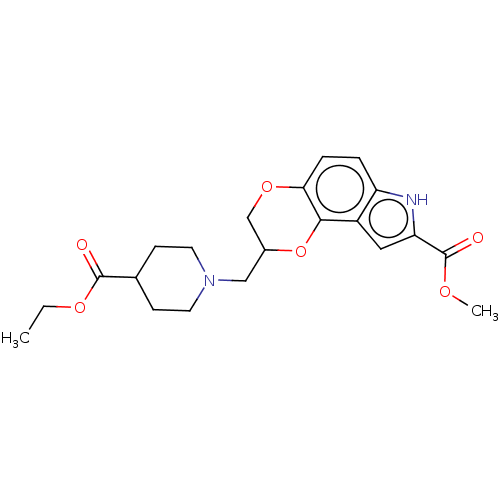 (8-(4-Ethoxycarbonyl-piperidin-1-ylmethyl)-7,8-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001119 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001115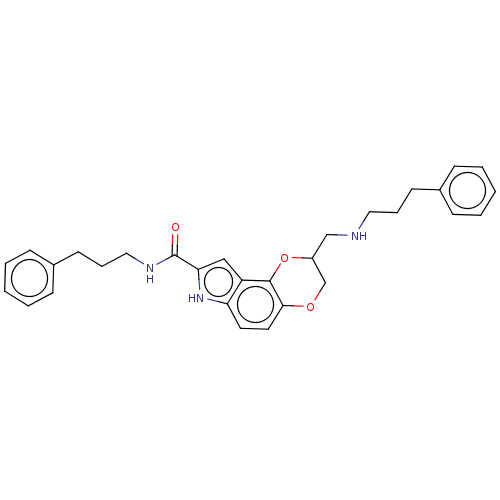 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001113 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001116 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001112 (8-(2-Hydroxymethyl-7,8-dihydro-3H-6,9-dioxa-3-aza-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001109 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001114 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using [3H]-U-86,17... | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001109 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards opioid receptor from rat whole brain using [3H]etorpine (33.2 Ci/mmol,0.2nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001112 (8-(2-Hydroxymethyl-7,8-dihydro-3H-6,9-dioxa-3-aza-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase II | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001116 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase II | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001109 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001110 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001119 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001114 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase II | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001116 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001112 (8-(2-Hydroxymethyl-7,8-dihydro-3H-6,9-dioxa-3-aza-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001114 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001115 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001111 (8-(4-Ethoxycarbonyl-piperidin-1-ylmethyl)-7,8-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001110 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase II | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001117 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001121 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001120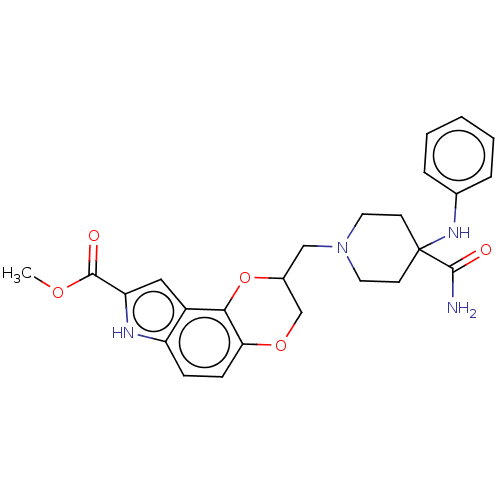 (8-(4-Carbamoyl-4-phenylamino-piperidin-1-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50001120 (8-(4-Carbamoyl-4-phenylamino-piperidin-1-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Displacement of [3H]-8-OH-DPAT from 5-hydroxytryptamine 1A receptor expressed in CHO cell membranes | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50001115 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor expressed in CHO cell membranes using [3H]ketanserin (60 Ci/mmol, 1 nM) | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001113 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001110 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Inhibition of human erythrocyte carbonic anhydrase II | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50001116 (8-[4-(2-Oxo-2,3-dihydro-benzoimidazol-1-yl)-piperi...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor expressed in CHO cell membranes using [3H]ketanserin (60 Ci/mmol, 1 nM) | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50001117 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor expressed in CHO cell membranes using [3H]ketanserin (60 Ci/mmol, 1 nM) | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001109 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Alpha-1 adrenergic receptor from rat whole brain using [3H]prazosin (18.1 Ci/mmol,0.9nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001115 (8-[(3-Phenyl-propylamino)-methyl]-7,8-dihydro-3H-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001121 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D2 from mammalian clones expressed in CHO cell membranes using 2 (86.1 Ci/mmol,1.7nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50001109 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor expressed in CHO cell membranes using [3H]ketanserin (60 Ci/mmol, 1 nM) | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1 (Rattus norvegicus (rat)-RAT) | BDBM50001121 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity towards opioid receptor from rat whole brain using [3H]etorpine (33.2 Ci/mmol,0.2nanoM)as radioligand | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50001110 (8-(4-Oxo-1-phenyl-1,3,8-triaza-spiro[4.5]dec-8-ylm...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor expressed in CHO cell membranes using [3H]ketanserin (60 Ci/mmol, 1 nM) | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50001111 (8-(4-Ethoxycarbonyl-piperidin-1-ylmethyl)-7,8-dihy...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor expressed in CHO cell membranes using [3H]ketanserin (60 Ci/mmol, 1 nM) | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (Homo sapiens (Human)) | BDBM50001120 (8-(4-Carbamoyl-4-phenylamino-piperidin-1-ylmethyl)...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2 receptor expressed in CHO cell membranes using [3H]ketanserin (60 Ci/mmol, 1 nM) | J Med Chem 35: 3058-66 (1992) BindingDB Entry DOI: 10.7270/Q21835G5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 61 total ) | Next | Last >> |