

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014015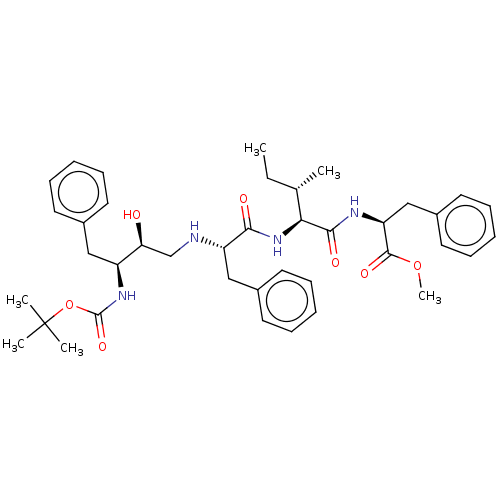 (2-{2-[2-(3-tert-Butoxycarbonylamino-2-hydroxy-4-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50010497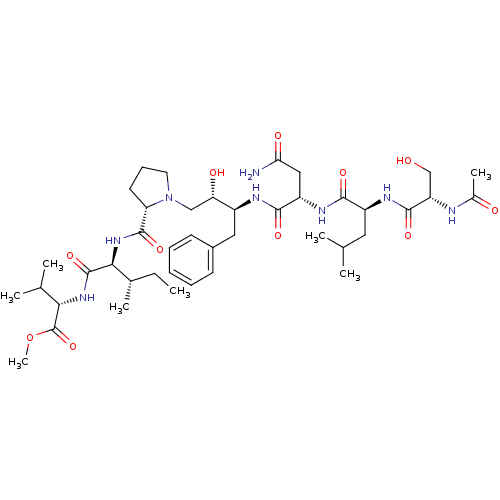 (Acetyl-Ser-Leu-Asn-Phe-[S]-[CH(OH)CH2N]Pro-Ile-Val...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig trachea using [3H]LTD4 | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014024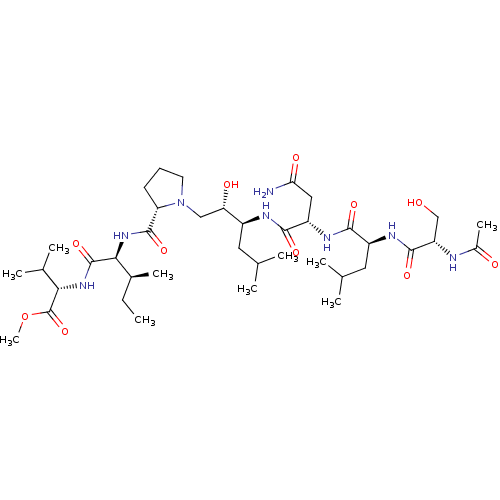 (2-(2-{[1-(2-{2-[2-(2-Acetylamino-3-hydroxy-propion...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibitory activity against HIV Protease was measured at pH 6.4 and 37 degrees C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014020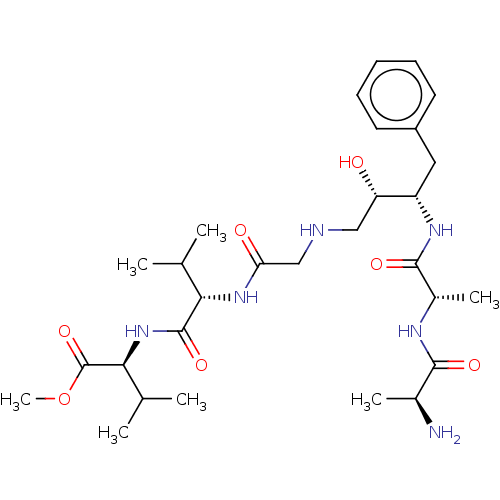 (2-[2-(2-{3-[2-(2-Amino-propionylamino)-propionylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014019 (2-{2-[(1-{2-[2-(2-Acetylamino-4-methyl-pentanoylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50421882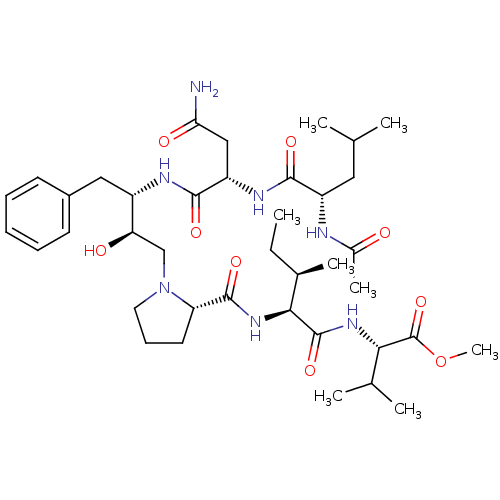 (CHEMBL2311136) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014014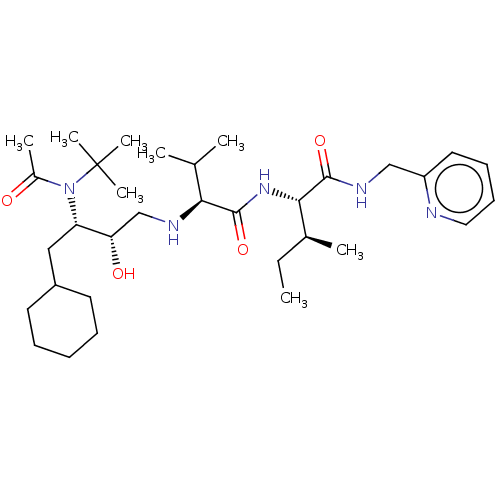 (2-{2-[3-(Acetyl-tert-butyl-amino)-4-cyclohexyl-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014028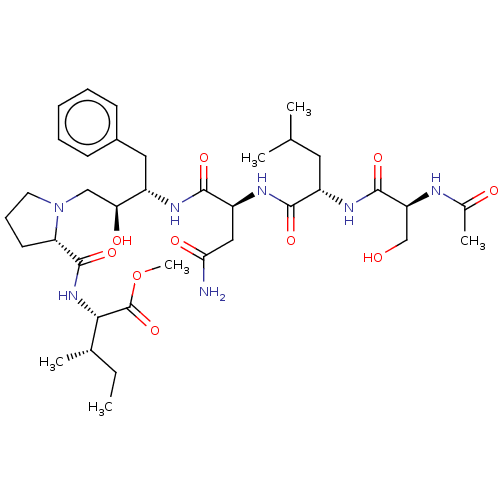 (2-{[1-(2-{2-[2-(2-Acetylamino-3-hydroxy-propionyla...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014023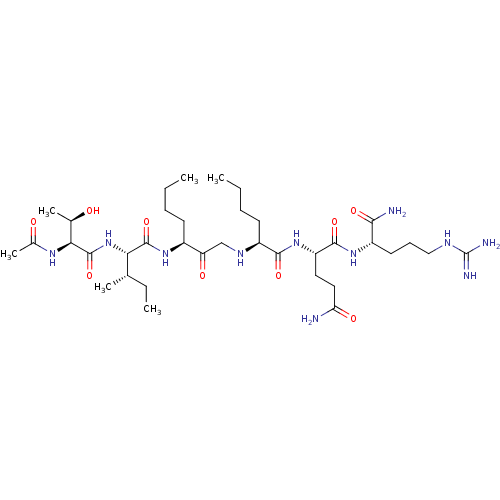 (2-(2-{3-[2-(2-Acetylamino-3-hydroxy-butyrylamino)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 780 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014026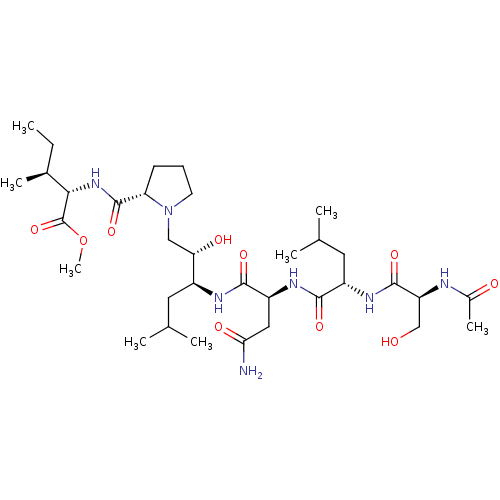 (2-{[1-(2-{2-[2-(2-Acetylamino-3-hydroxy-propionyla...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 980 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibitory activity against HIV Protease was measured at pH 6.4 and 37 degrees C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50000902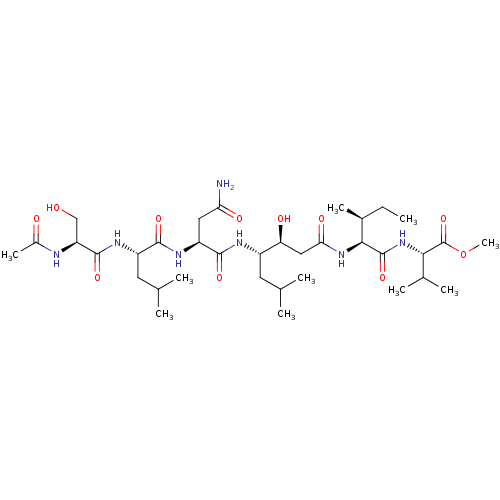 (CHEMBL52494) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50000543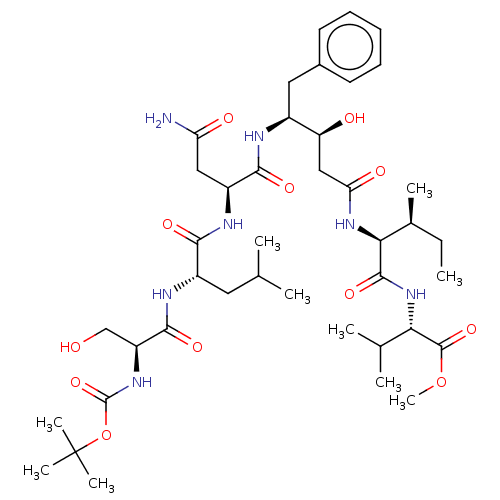 (CHEMBL417012) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014018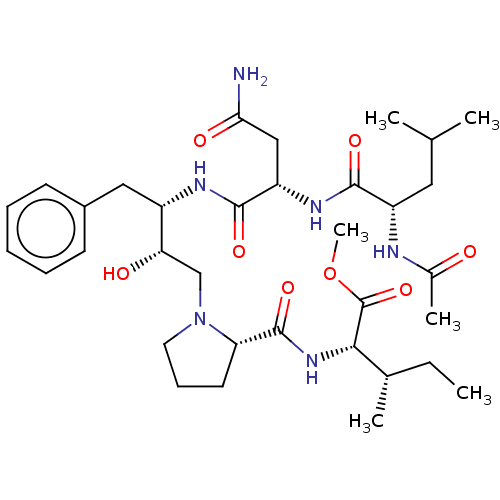 (2-[(1-{2-[2-(2-Acetylamino-4-methyl-pentanoylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50000550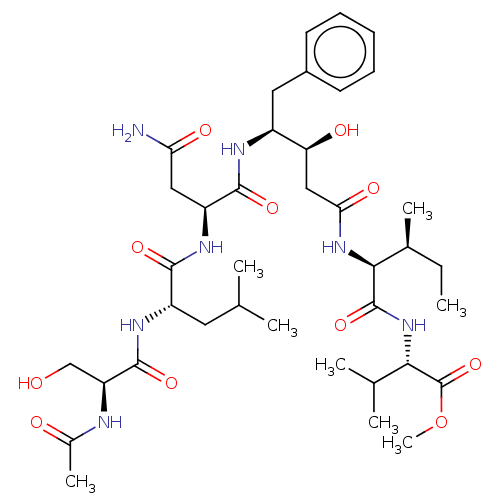 (CHEMBL298903) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibitory activity against HIV Protease was measured at pH 6.4 and 37 degrees C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014021 (2-[(1-{2-[2-(2-Acetylamino-4-methyl-pentanoylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibitory activity against HIV Protease was measured at pH 6.4 and 37 degrees C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50014030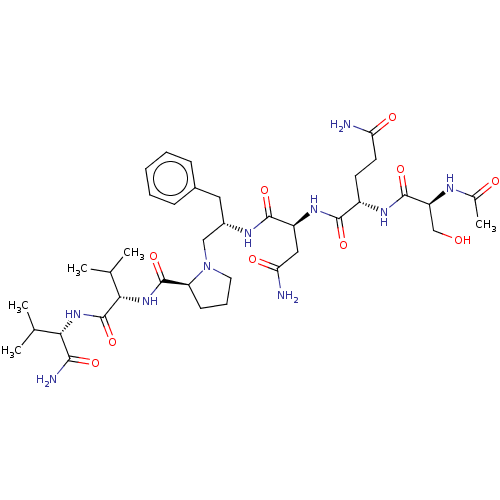 (2-(2-Acetylamino-3-hydroxy-propionylamino)-pentane...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50000698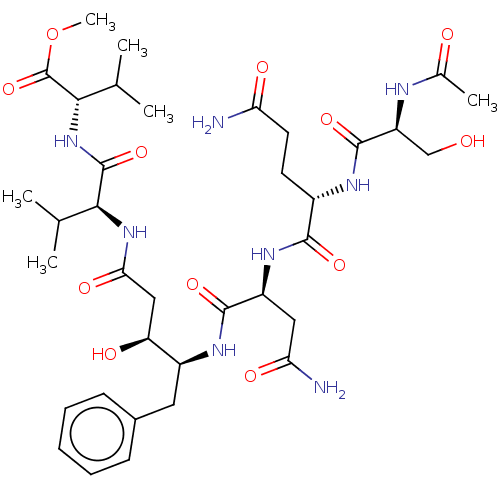 (CHEMBL49026) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.4 | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Inhibition of HIV protease at pH 6.4, 37 degree C | J Med Chem 33: 1285-8 (1990) BindingDB Entry DOI: 10.7270/Q2SB44QS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||