 Found 18 hits Enz. Inhib. hit(s) with all data for entry = 50004739
Found 18 hits Enz. Inhib. hit(s) with all data for entry = 50004739 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13014
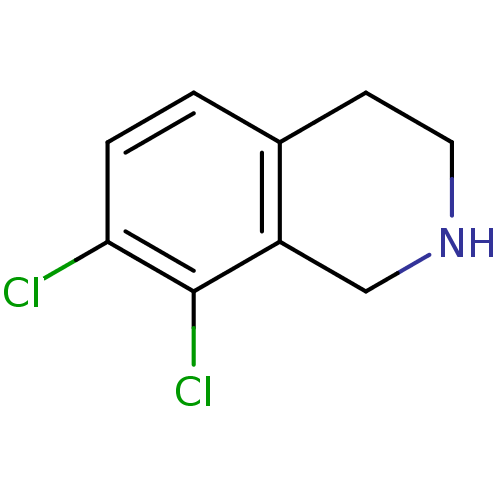
(7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...)Show InChI InChI=1S/C9H9Cl2N/c10-8-2-1-6-3-4-12-5-7(6)9(8)11/h1-2,12H,3-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029108

(6,7,8-Trichloro-1,2,3,4-tetrahydro-isoquinoline | ...)Show InChI InChI=1S/C9H8Cl3N/c10-7-3-5-1-2-13-4-6(5)8(11)9(7)12/h3,13H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029102

(7-Chloro-1,2,3,4-tetrahydro-isoquinoline | CHEMBL1...)Show InChI InChI=1S/C9H10ClN/c10-9-2-1-7-3-4-11-6-8(7)5-9/h1-2,5,11H,3-4,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029107

(8-Chloro-1,2,3,4-tetrahydro-isoquinoline | CHEMBL1...)Show InChI InChI=1S/C9H10ClN/c10-9-3-1-2-7-4-5-11-6-8(7)9/h1-3,11H,4-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029101
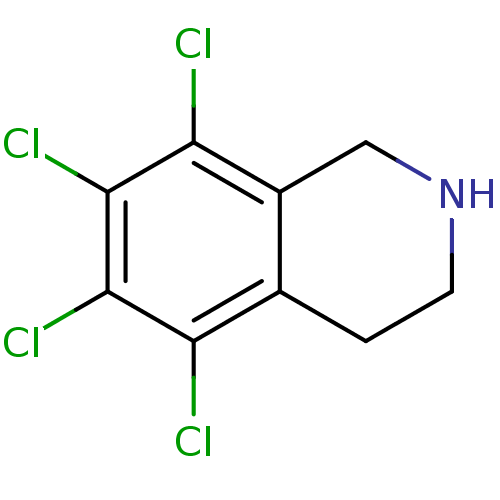
(5,6,7,8-Tetrachloro-1,2,3,4-tetrahydro-isoquinolin...)Show InChI InChI=1S/C9H7Cl4N/c10-6-4-1-2-14-3-5(4)7(11)9(13)8(6)12/h14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029099

(5,7,8-Trichloro-1,2,3,4-tetrahydro-isoquinoline | ...)Show InChI InChI=1S/C9H8Cl3N/c10-7-3-8(11)9(12)6-4-13-2-1-5(6)7/h3,13H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM13014

(7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...)Show InChI InChI=1S/C9H9Cl2N/c10-8-2-1-6-3-4-12-5-7(6)9(8)11/h1-2,12H,3-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure PNMT-inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029108

(6,7,8-Trichloro-1,2,3,4-tetrahydro-isoquinoline | ...)Show InChI InChI=1S/C9H8Cl3N/c10-7-3-5-1-2-13-4-6(5)8(11)9(7)12/h3,13H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029101

(5,6,7,8-Tetrachloro-1,2,3,4-tetrahydro-isoquinolin...)Show InChI InChI=1S/C9H7Cl4N/c10-6-4-1-2-14-3-5(4)7(11)9(13)8(6)12/h14H,1-3H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029107

(8-Chloro-1,2,3,4-tetrahydro-isoquinoline | CHEMBL1...)Show InChI InChI=1S/C9H10ClN/c10-9-3-1-2-7-4-5-11-6-8(7)9/h1-3,11H,4-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029102

(7-Chloro-1,2,3,4-tetrahydro-isoquinoline | CHEMBL1...)Show InChI InChI=1S/C9H10ClN/c10-9-2-1-7-3-4-11-6-8(7)5-9/h1-2,5,11H,3-4,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029099

(5,7,8-Trichloro-1,2,3,4-tetrahydro-isoquinoline | ...)Show InChI InChI=1S/C9H8Cl3N/c10-7-3-8(11)9(12)6-4-13-2-1-5(6)7/h3,13H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Dissociation constant(Ki) of compound was determined to measure Phenylethanolamine N-methyltransferase inhibitory potency |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029106

(2,3-Dichloro-benzylamine | CHEMBL13165)Show InChI InChI=1S/C7H7Cl2N/c8-6-3-1-2-5(4-10)7(6)9/h1-3H,4,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM12584

((3-chlorophenyl)methanamine | 1-(3-CHLOROPHENYL)ME...)Show InChI InChI=1S/C7H8ClN/c8-7-3-1-2-6(4-7)5-9/h1-4H,5,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029105
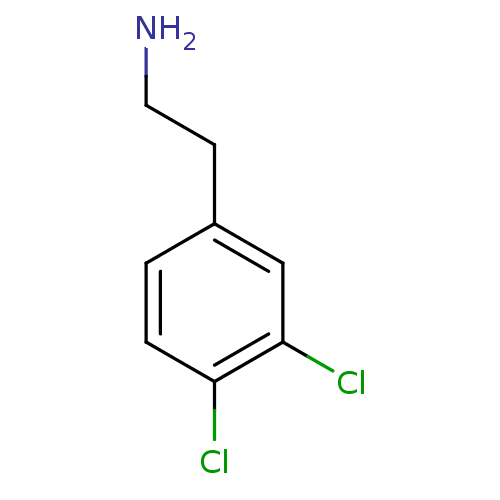
(2-(3,4,-dichlorophenyl)ethylamine | 2-(3,4-Dichlor...)Show InChI InChI=1S/C8H9Cl2N/c9-7-2-1-6(3-4-11)5-8(7)10/h1-2,5H,3-4,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029104
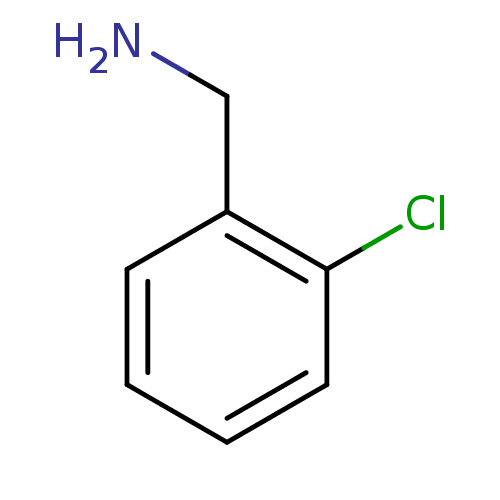
(2-Chloro-benzylamine | CHEMBL12712)Show InChI InChI=1S/C7H8ClN/c8-7-4-2-1-3-6(7)5-9/h1-4H,5,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029103

(2-(3-Chloro-phenyl)-1-methyl-ethylamine | CHEMBL14...)Show InChI InChI=1S/C9H12ClN/c1-7(11)5-8-3-2-4-9(10)6-8/h2-4,6-7H,5,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |
Phenylethanolamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50029100
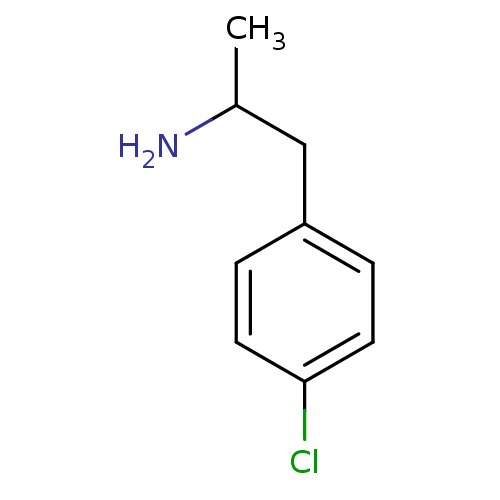
(2-(4-Chloro-phenyl)-1-methyl-ethylamine | CHEMBL35...)Show InChI InChI=1S/C9H12ClN/c1-7(11)6-8-2-4-9(10)5-3-8/h2-5,7H,6,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Invitro inhibitory potency of the compound was measured against phenylethanolamine N-methyltransferase |
J Med Chem 23: 506-11 (1980)
BindingDB Entry DOI: 10.7270/Q2F47N5S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data