

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054718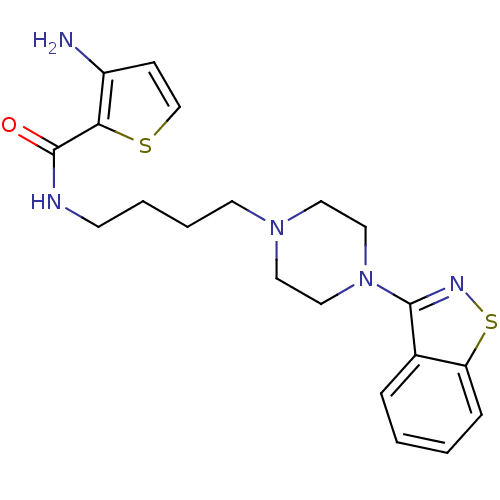 (3-Amino-thiophene-2-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054710 (1,2,3,4-Tetrahydro-quinoline-8-carboxylic acid [4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054713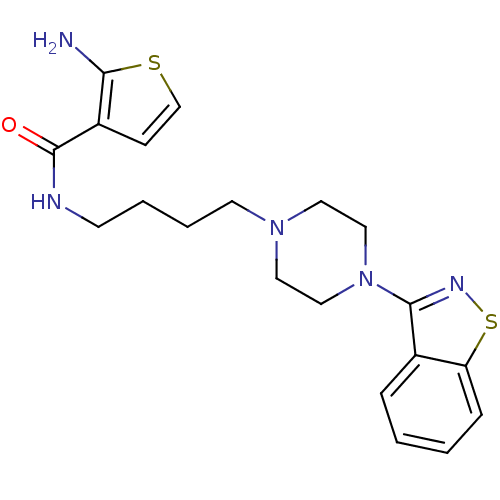 (2-Amino-thiophene-3-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054716 (1H-Indazole-3-carboxylic acid [4-(4-benzo[d]isothi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054706 (1H-Indole-7-carboxylic acid [4-(4-benzo[d]isothiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054707 (CHEMBL344994 | Pyridine-2-carboxylic acid [4-(4-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054705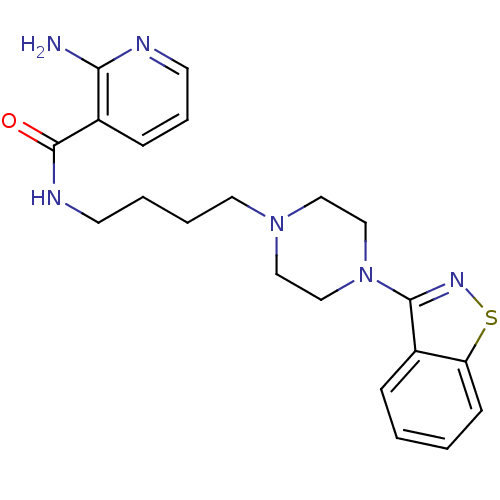 (2-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054715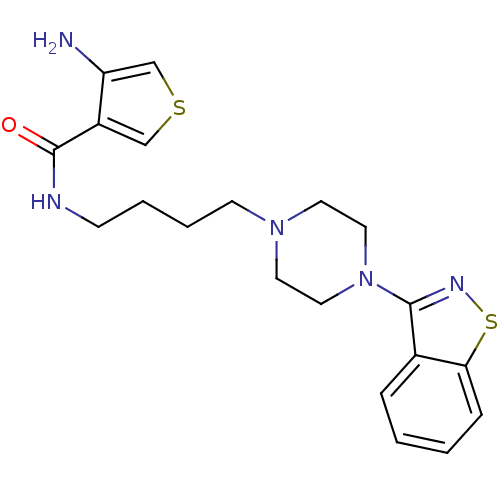 (4-Amino-thiophene-3-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054716 (1H-Indazole-3-carboxylic acid [4-(4-benzo[d]isothi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054714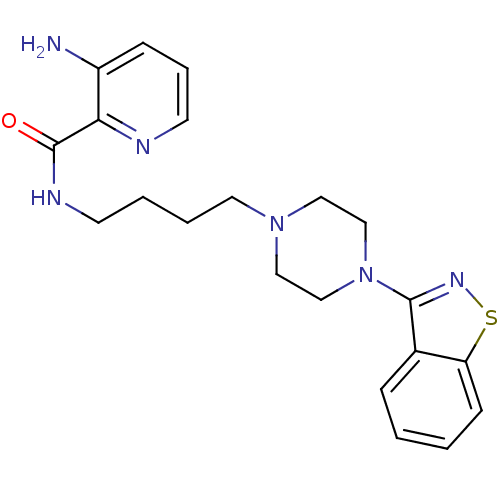 (3-Amino-pyridine-2-carboxylic acid [4-(4-benzo[d]i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054712 (3H-Benzoimidazole-4-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054704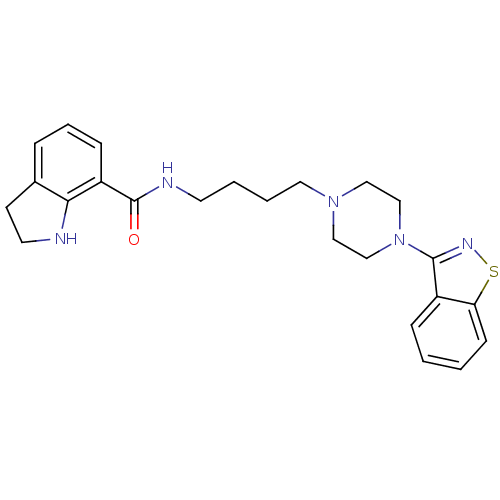 (2,3-Dihydro-1H-indole-7-carboxylic acid [4-(4-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054703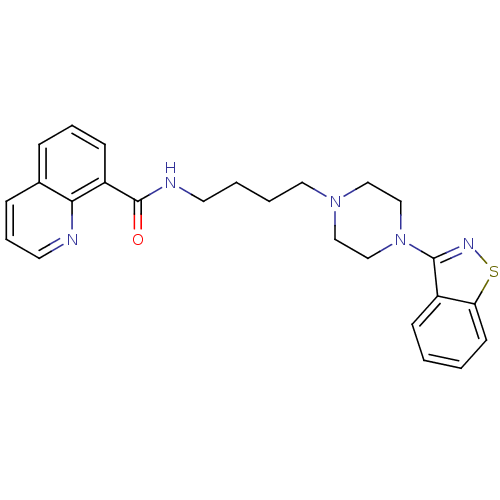 (CHEMBL358268 | Quinoline-8-carboxylic acid [4-(4-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054711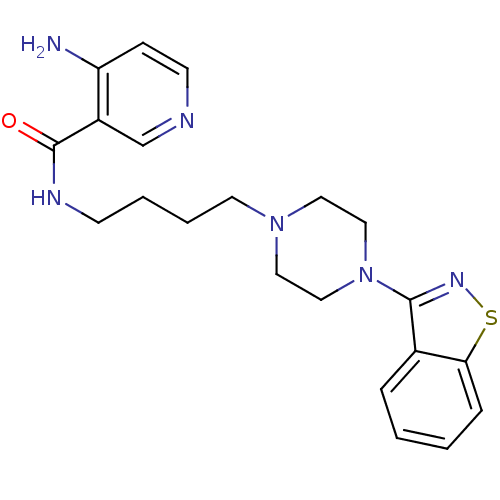 (4-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 from mice | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054719 (CHEMBL356498 | N-[4-(4-Benzo[d]isothiazol-3-yl-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054711 (4-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054708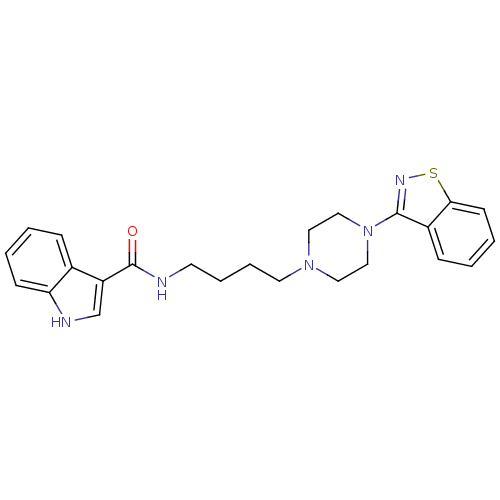 (1H-Indole-3-carboxylic acid [4-(4-benzo[d]isothiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054702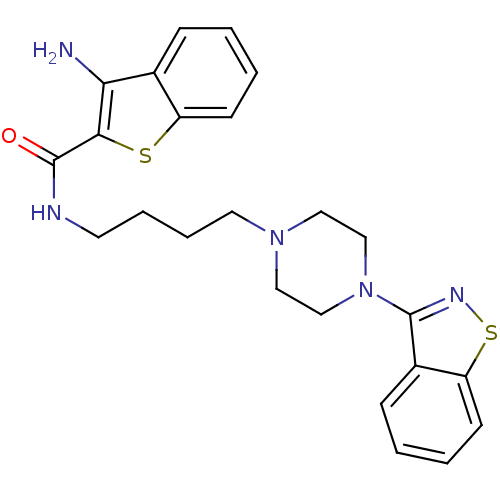 (3-Amino-benzo[b]thiophene-2-carboxylic acid [4-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054702 (3-Amino-benzo[b]thiophene-2-carboxylic acid [4-(4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054715 (4-Amino-thiophene-3-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054709 (CHEMBL141494 | N-[4-(4-Benzo[d]isothiazol-3-yl-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50054717 (3-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054712 (3H-Benzoimidazole-4-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 from mice | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054705 (2-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054718 (3-Amino-thiophene-2-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054713 (2-Amino-thiophene-3-carboxylic acid [4-(4-benzo[d]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 from mice | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054708 (1H-Indole-3-carboxylic acid [4-(4-benzo[d]isothiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 from mice | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054703 (CHEMBL358268 | Quinoline-8-carboxylic acid [4-(4-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 from mice | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054717 (3-Amino-N-[4-(4-benzo[d]isothiazol-3-yl-piperazin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 from mice | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054707 (CHEMBL344994 | Pyridine-2-carboxylic acid [4-(4-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054714 (3-Amino-pyridine-2-carboxylic acid [4-(4-benzo[d]i...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 from mice | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054709 (CHEMBL141494 | N-[4-(4-Benzo[d]isothiazol-3-yl-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Dopamine receptor D2 from mice | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054706 (1H-Indole-7-carboxylic acid [4-(4-benzo[d]isothiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054710 (1,2,3,4-Tetrahydro-quinoline-8-carboxylic acid [4-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054704 (2,3-Dihydro-1H-indole-7-carboxylic acid [4-(4-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50054719 (CHEMBL356498 | N-[4-(4-Benzo[d]isothiazol-3-yl-pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Mus musculus (Mouse)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibition of mouse Dopamine receptor D2 | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50001884 (2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Inc. Curated by ChEMBL | Assay Description Inhibitory activity against serotonin 5-hydroxytryptamine 1A receptor from mice. | J Med Chem 39: 4692-703 (1997) Article DOI: 10.1021/jm9603375 BindingDB Entry DOI: 10.7270/Q2VT1R6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||