

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035018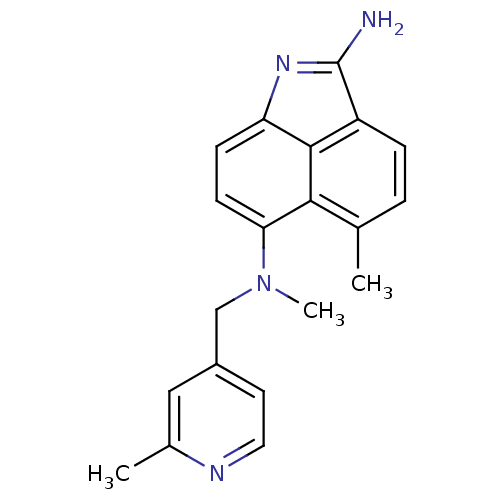 (5,N*6*-Dimethyl-N*6*-(2-methyl-pyridin-4-ylmethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035012 (5,N*6*-Dimethyl-N*6*-pyridazin-4-ylmethyl-benzo[cd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035013 (5,N*2*,N*6*-Trimethyl-N*6*-pyridin-4-ylmethyl-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035006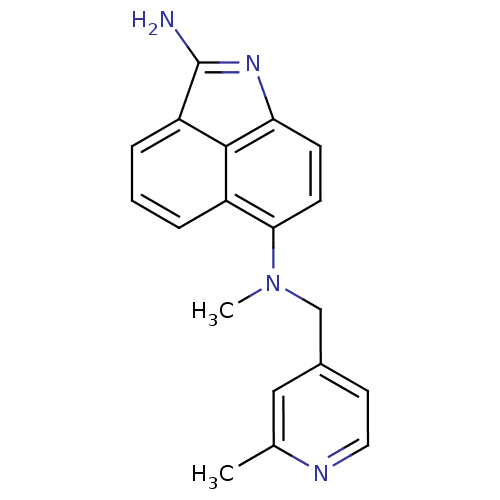 (CHEMBL434980 | N*6*-Methyl-N*6*-(2-methyl-pyridin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035009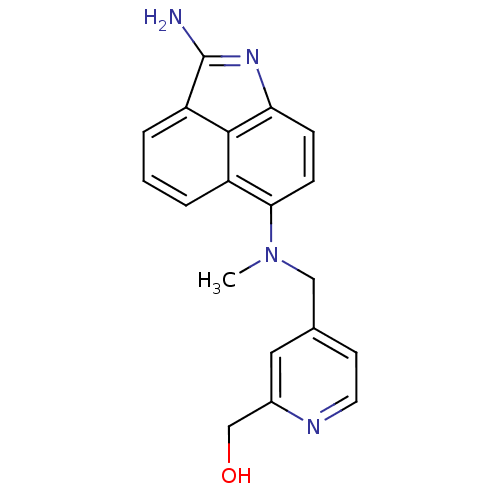 ((4-{[(2-Amino-benzo[cd]indol-6-yl)-methyl-amino]-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035005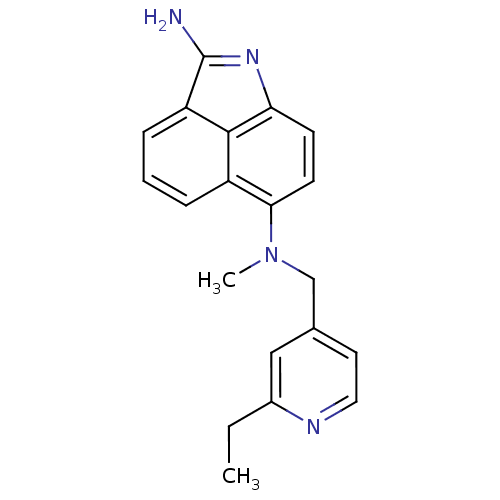 (CHEMBL56611 | N*6*-(2-Ethyl-pyridin-4-ylmethyl)-N*...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035007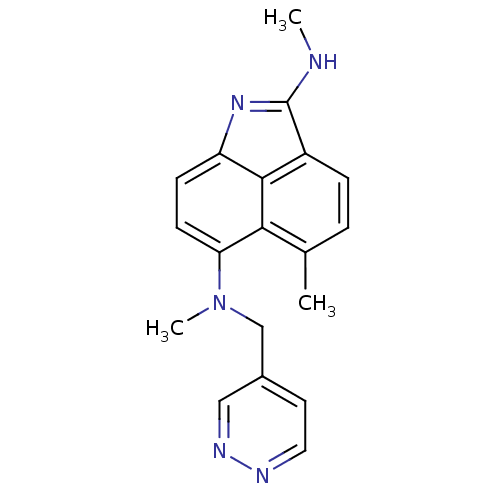 (5,N*2*,N*6*-Trimethyl-N*6*-pyridazin-4-ylmethyl-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035011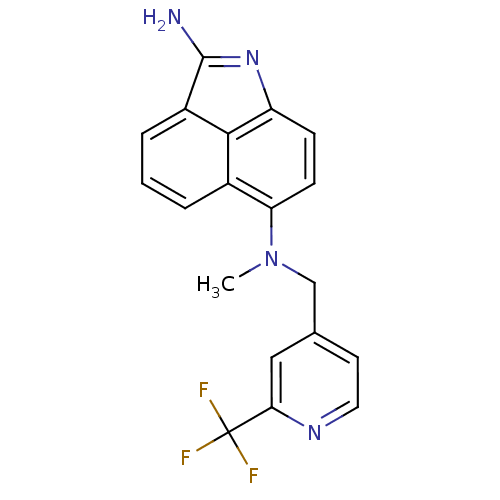 (CHEMBL301218 | N*6*-Methyl-N*6*-(2-trifluoromethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035014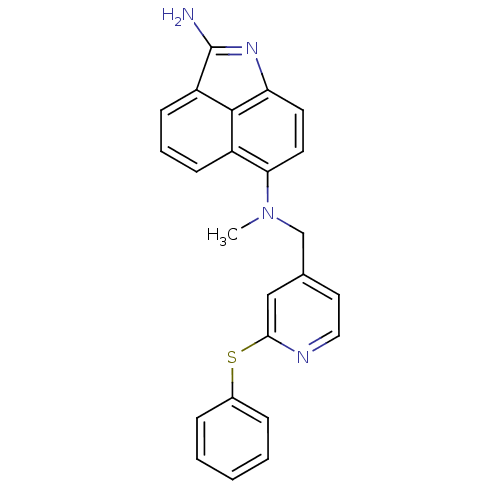 (CHEMBL56637 | N*6*-Methyl-N*6*-(2-phenylsulfanyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035010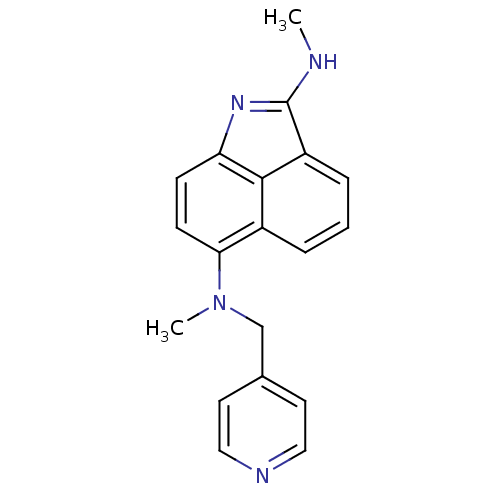 (CHEMBL55361 | N*2*,N*6*-Dimethyl-N*6*-pyridin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035008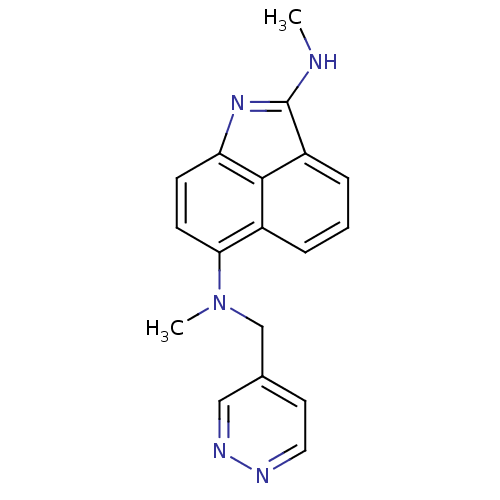 (CHEMBL417763 | N*2*,N*6*-Dimethyl-N*6*-pyridazin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect against recombinant human thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50035017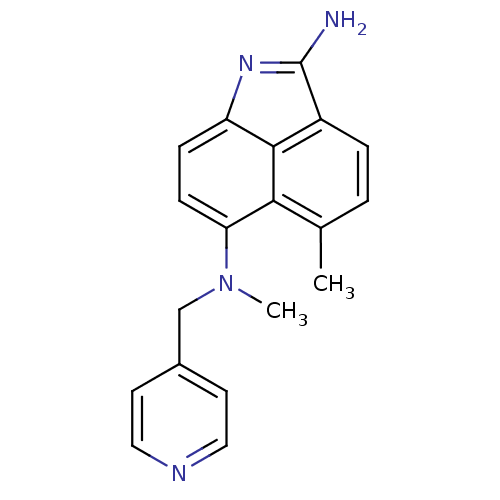 (5,N*6*-Dimethyl-N*6*-pyridin-4-ylmethyl-benzo[cd]i...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035017 (5,N*6*-Dimethyl-N*6*-pyridin-4-ylmethyl-benzo[cd]i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50035006 (CHEMBL434980 | N*6*-Methyl-N*6*-(2-methyl-pyridin-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50005210 ((2-Imino-1-methyl-1,2-dihydro-benzo[cd]indol-6-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50005210 ((2-Imino-1-methyl-1,2-dihydro-benzo[cd]indol-6-yl)...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50035015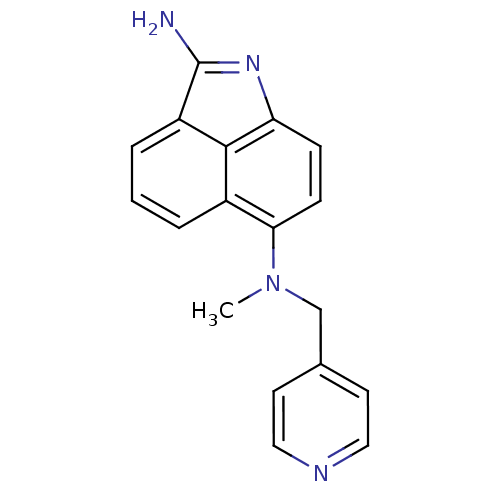 (CHEMBL55884 | N*6*-Methyl-N*6*-pyridin-4-ylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035015 (CHEMBL55884 | N*6*-Methyl-N*6*-pyridin-4-ylmethyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50035016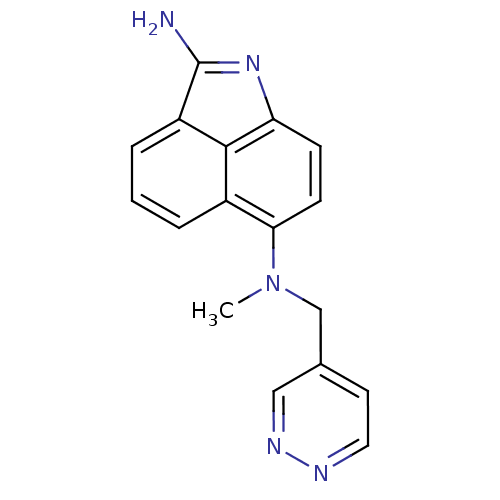 (CHEMBL293823 | N*6*-Methyl-N*6*-pyridazin-4-ylmeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Escherichia coli) | BDBM50035016 (CHEMBL293823 | N*6*-Methyl-N*6*-pyridazin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory effect of the compound against E. coli thymidylate synthase | J Med Chem 38: 1892-903 (1995) BindingDB Entry DOI: 10.7270/Q2X929B2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||