

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048509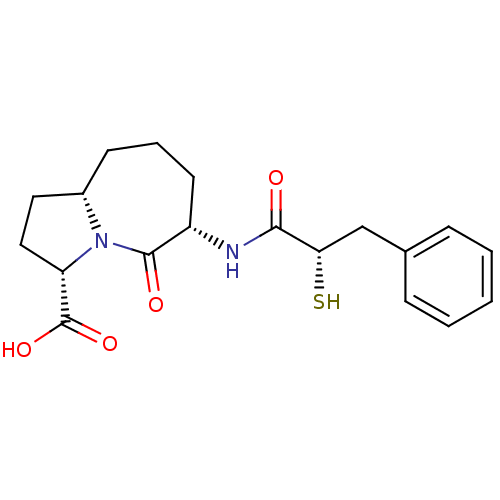 ((3S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048505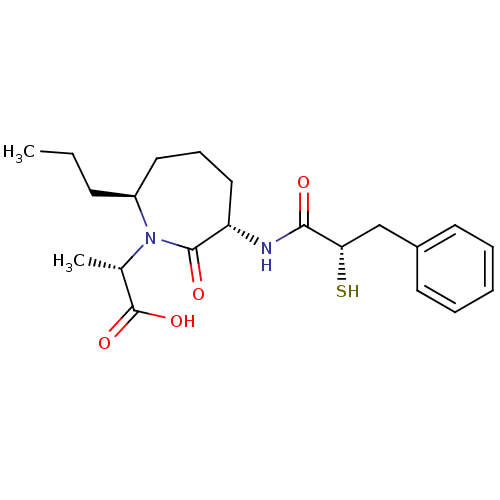 ((S)-2-[(3S,7S)-3-((S)-2-Mercapto-3-phenyl-propiony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048512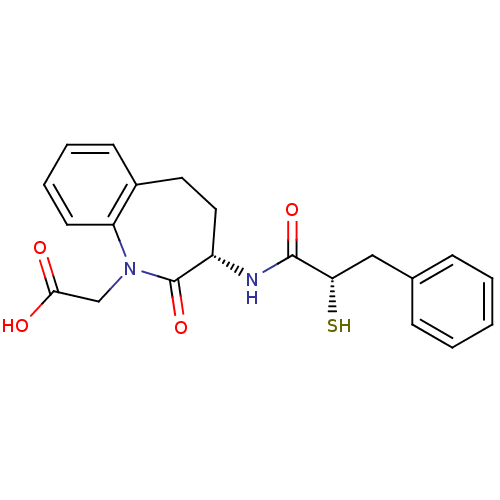 (CHEMBL299639 | [(S)-3-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048504 (CHEMBL148155 | [(3S,7R)-7-Allyl-3-((S)-2-mercapto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048506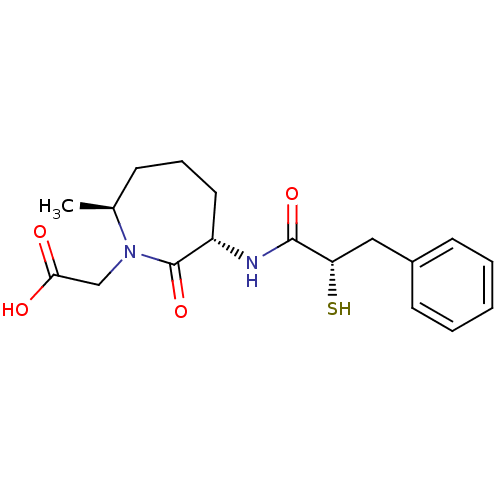 (CHEMBL104054 | [(3S,7S)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048515 (CHEMBL345021 | [(3S,7R)-7-Isobutyl-3-((S)-2-mercap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048513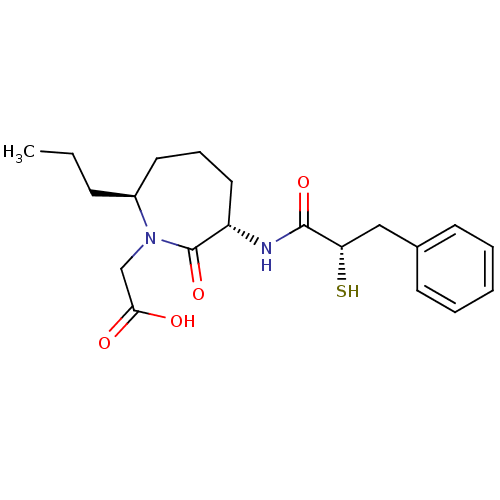 (CHEMBL147043 | [(3S,7S)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048500 ((4S,7S,10aS)-7-((S)-2-Mercapto-3-phenyl-propionyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048512 (CHEMBL299639 | [(S)-3-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048502 (CHEMBL343191 | [(3S,7R)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048517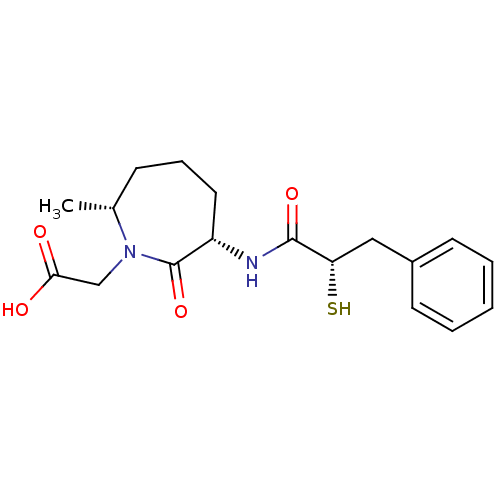 (CHEMBL318096 | [(3S,7R)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048505 ((S)-2-[(3S,7S)-3-((S)-2-Mercapto-3-phenyl-propiony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048508 (CHEMBL147153 | [(3S,7R)-7-Cyclopentyl-3-((S)-2-mer...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048509 ((3S,6S,9aS)-6-((S)-2-Mercapto-3-phenyl-propionylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048507 (CHEMBL299169 | [(S)-3-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048501 (CHEMBL146823 | [(3S,7R)-7-Cyclopropylmethyl-3-((S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048511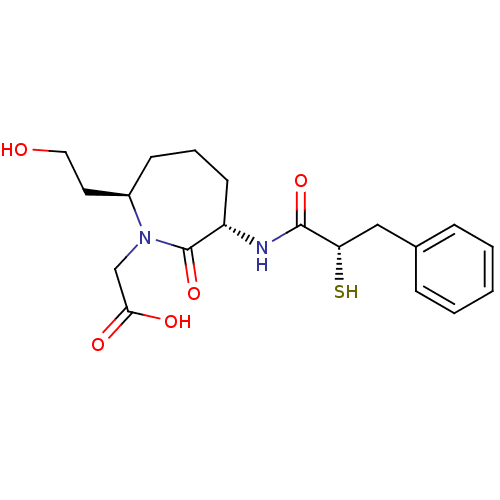 (CHEMBL146371 | [(3S,7R)-7-(2-Hydroxy-ethyl)-3-((S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048516 ((S)-2-[(3S,7S)-3-((S)-2-Mercapto-3-phenyl-propiony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048500 ((4S,7S,10aS)-7-((S)-2-Mercapto-3-phenyl-propionyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048510 ((S)-2-[(S)-3-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048514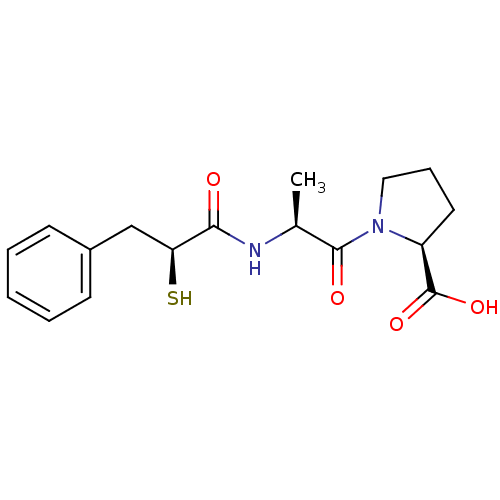 ((S)-1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048511 (CHEMBL146371 | [(3S,7R)-7-(2-Hydroxy-ethyl)-3-((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048518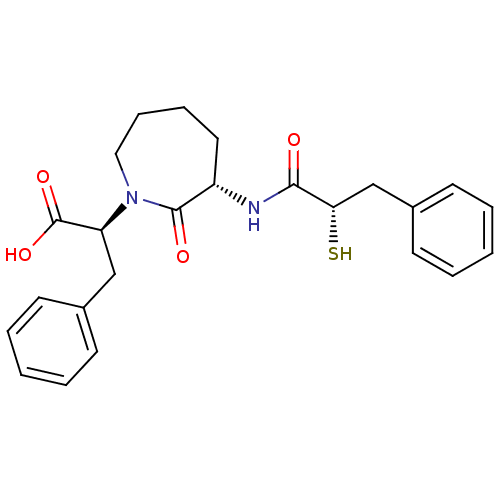 ((S)-2-[(S)-3-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048513 (CHEMBL147043 | [(3S,7S)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048506 (CHEMBL104054 | [(3S,7S)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048504 (CHEMBL148155 | [(3S,7R)-7-Allyl-3-((S)-2-mercapto-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048507 (CHEMBL299169 | [(S)-3-((S)-2-Mercapto-3-phenyl-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048516 ((S)-2-[(3S,7S)-3-((S)-2-Mercapto-3-phenyl-propiony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048510 ((S)-2-[(S)-3-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048501 (CHEMBL146823 | [(3S,7R)-7-Cyclopropylmethyl-3-((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048517 (CHEMBL318096 | [(3S,7R)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048514 ((S)-1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048502 (CHEMBL343191 | [(3S,7R)-3-((S)-2-Mercapto-3-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 466 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048518 ((S)-2-[(S)-3-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 479 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50048503 ((S)-2-[(S)-3-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 907 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme isolated from rabbit lung extract. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048503 ((S)-2-[(S)-3-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048508 (CHEMBL147153 | [(3S,7R)-7-Cyclopentyl-3-((S)-2-mer...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50048515 (CHEMBL345021 | [(3S,7R)-7-Isobutyl-3-((S)-2-mercap...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of purified rat kidney Neutral endopeptidase. | J Med Chem 39: 494-502 (1996) Article DOI: 10.1021/jm950677a BindingDB Entry DOI: 10.7270/Q2GF0SM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||