

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acrosin (Homo sapiens (Human)) | BDBM50018974 (CHEMBL3287446) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018973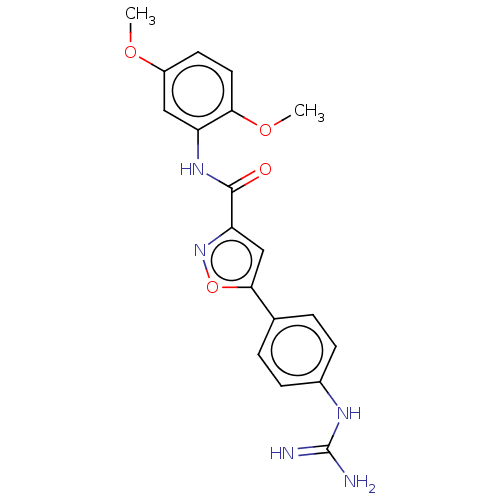 (CHEMBL3287445) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018978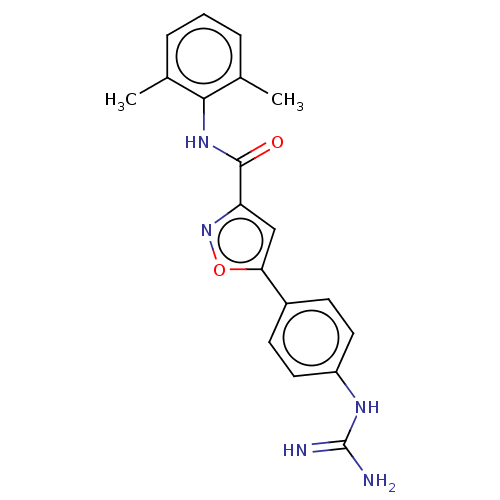 (CHEMBL3287450) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018983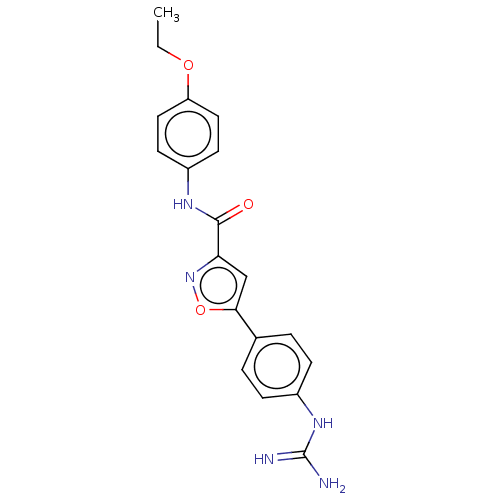 (CHEMBL3287455) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018988 (CHEMBL3287459) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018976 (CHEMBL3287448) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018987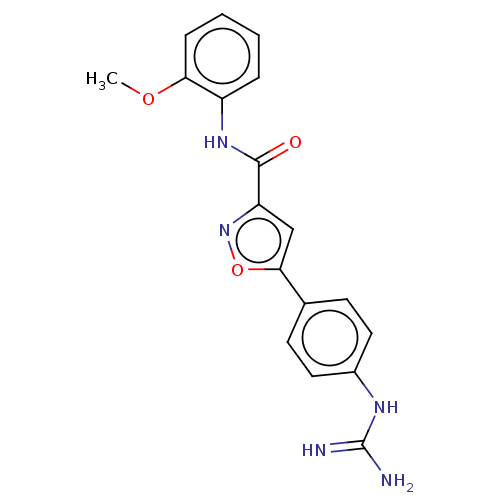 (CHEMBL3287458) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018975 (CHEMBL3287447) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018992 (CHEMBL3287462) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018977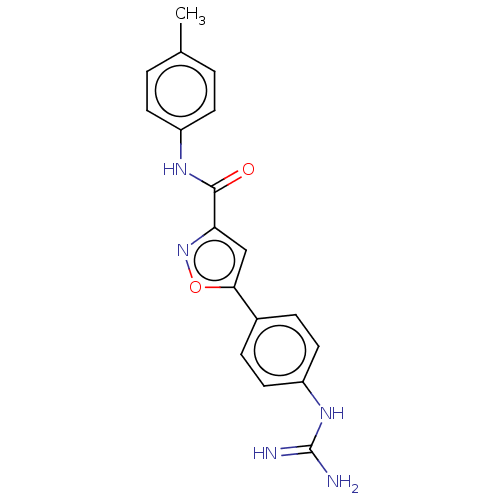 (CHEMBL3287449) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018994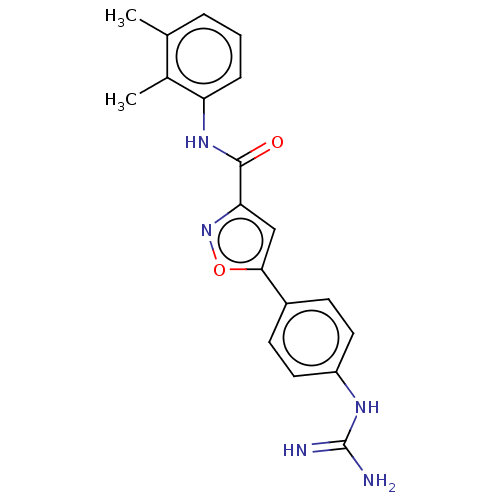 (CHEMBL3287464) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018980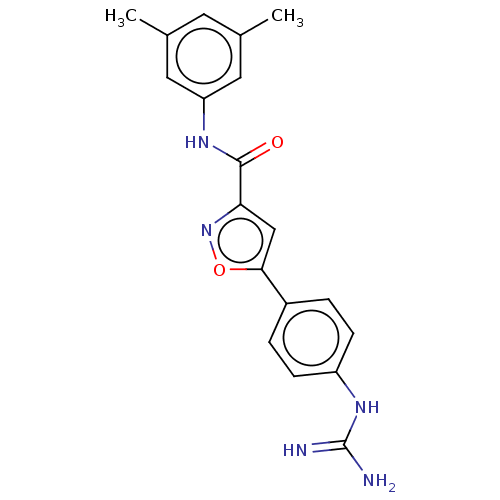 (CHEMBL3287452) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018982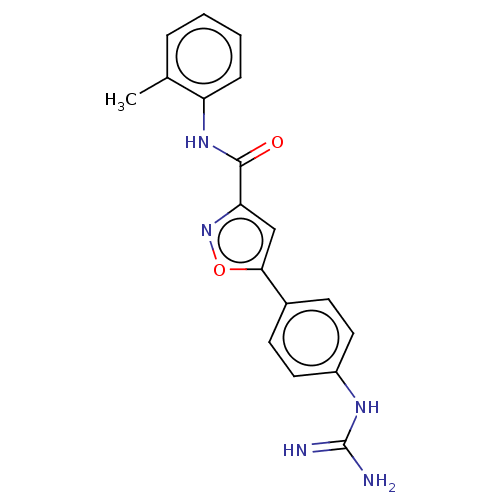 (CHEMBL3287454) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018981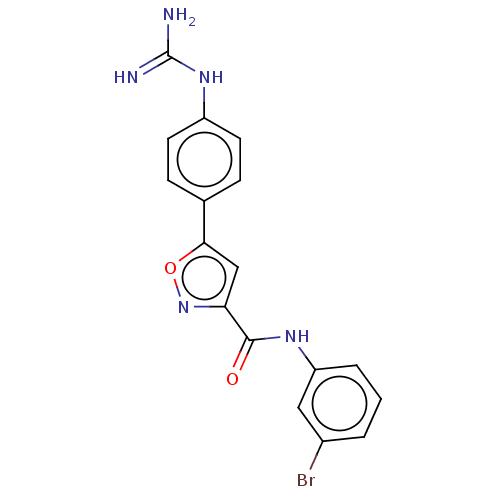 (CHEMBL3287453) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018984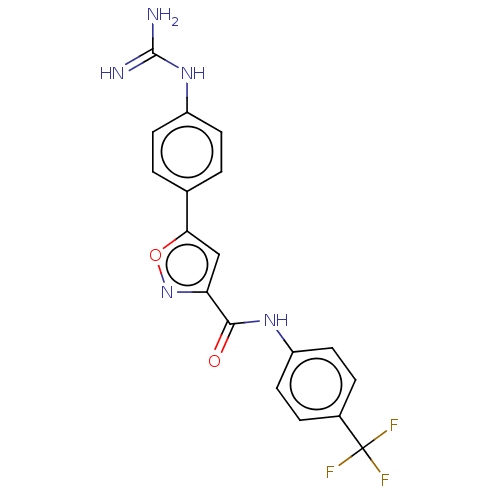 (CHEMBL3287456) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018995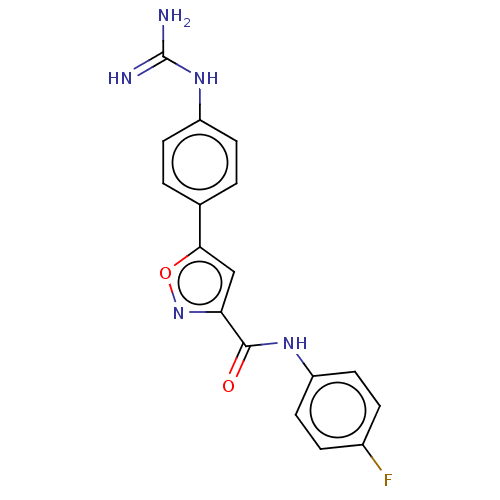 (CHEMBL3287465) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018972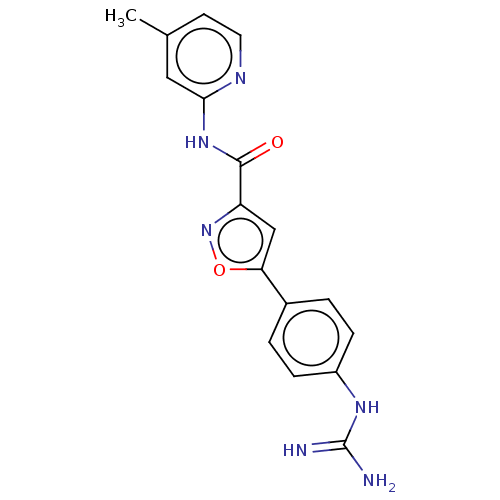 (CHEMBL3287444) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018993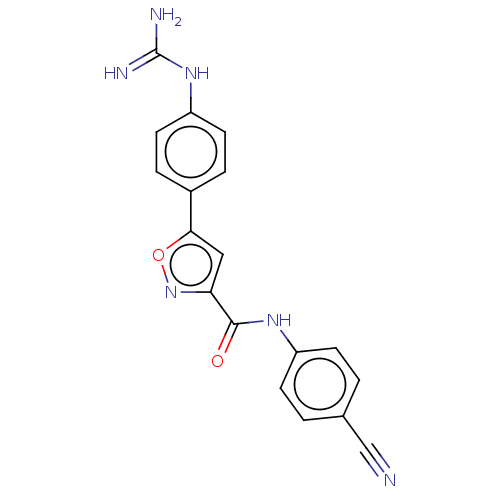 (CHEMBL3287463) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018971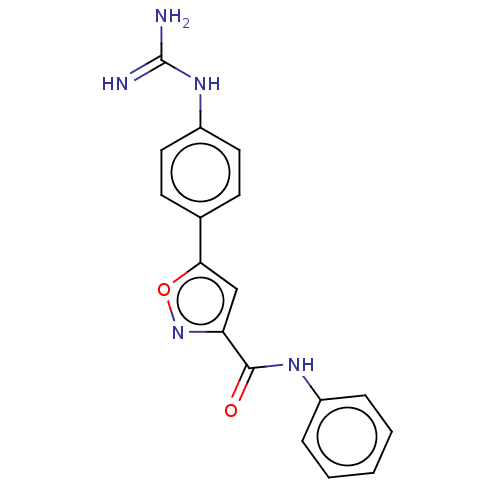 (CHEMBL3287443) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018979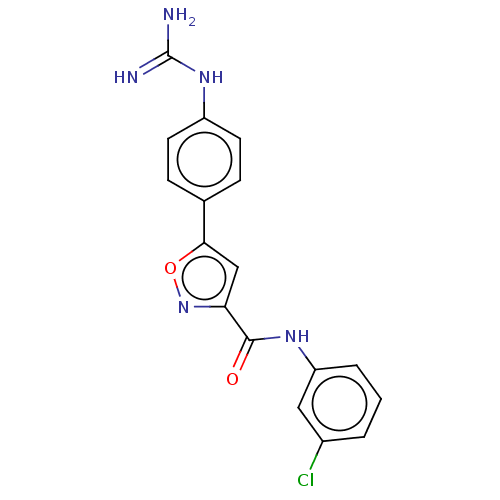 (CHEMBL3287451) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018986 (CHEMBL3287457) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018989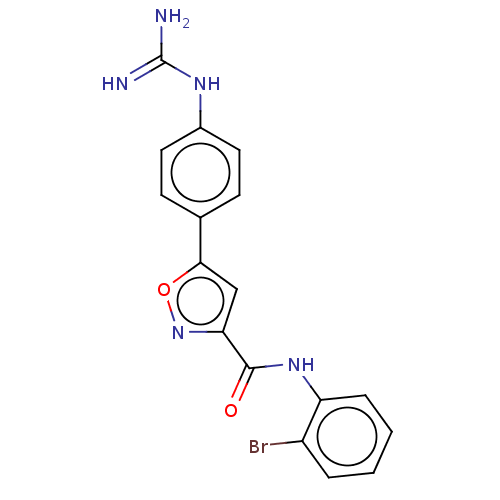 (CHEMBL3287460) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018990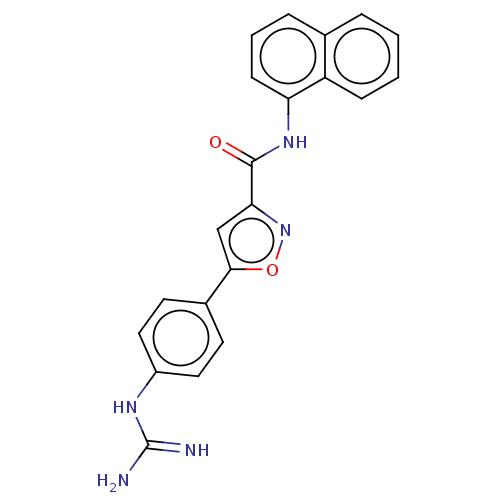 (CHEMBL3287461) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018965 (CHEMBL3287437) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018970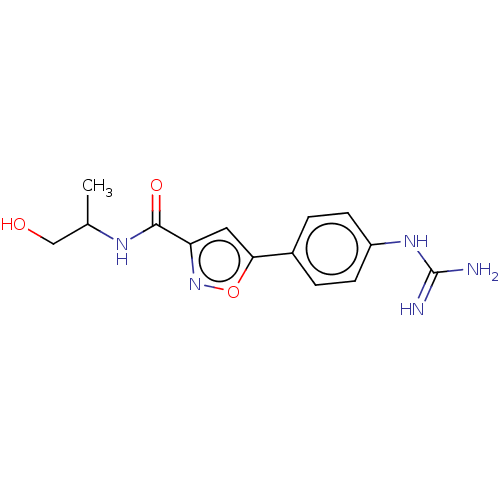 (CHEMBL3287442) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018969 (CHEMBL3287441) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018966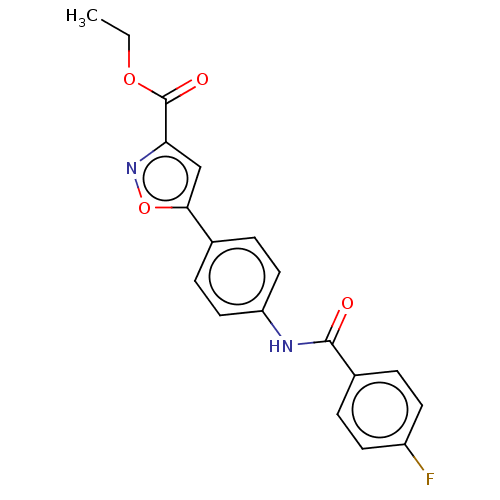 (CHEMBL3287438) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018967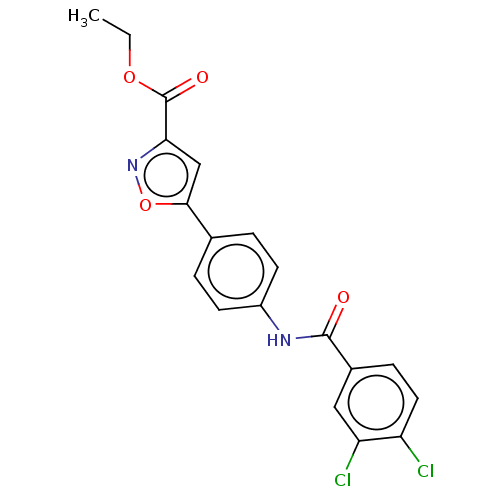 (CHEMBL3287439) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018968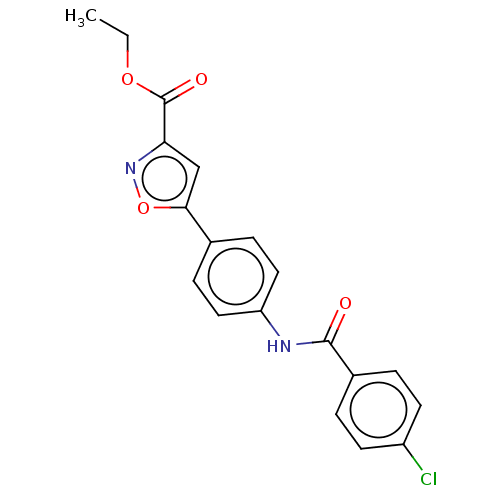 (CHEMBL3287440) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50382109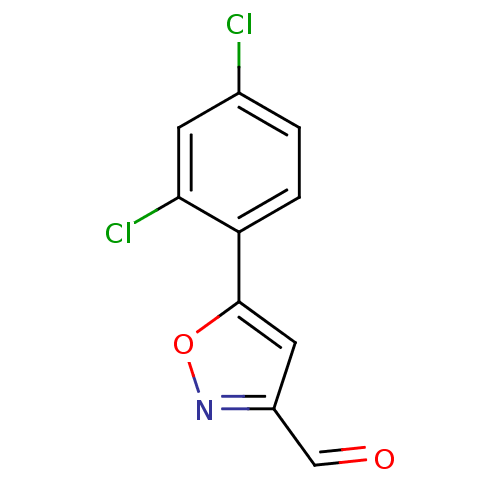 (CHEMBL2023319) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acrosin (Homo sapiens (Human)) | BDBM50018964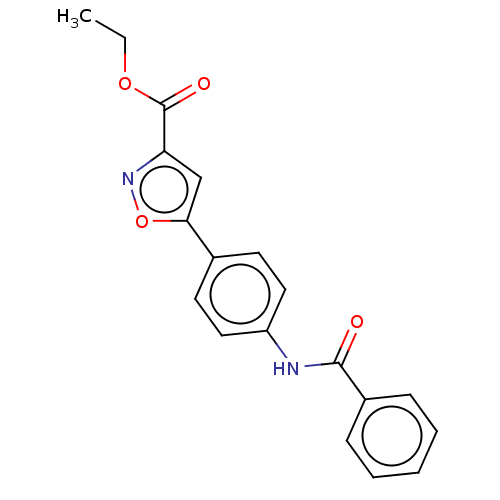 (CHEMBL3287436) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Second Military Medical University Curated by ChEMBL | Assay Description Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometry | Bioorg Med Chem Lett 24: 2802-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.118 BindingDB Entry DOI: 10.7270/Q2J38V4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||