 Found 19 hits Enz. Inhib. hit(s) with all data for entry = 50029306
Found 19 hits Enz. Inhib. hit(s) with all data for entry = 50029306 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283145
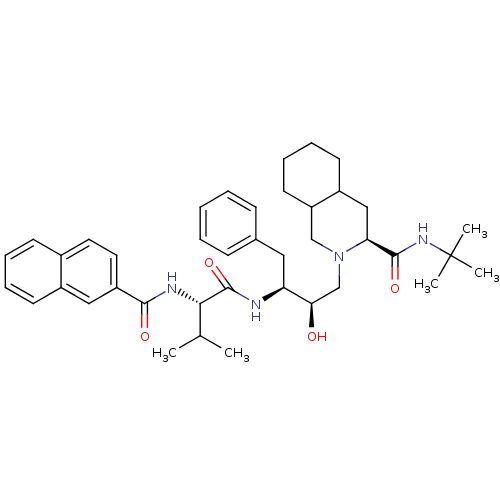
((4S,4aS,5S)-2-((2R,3S)-2-Hydroxy-3-{3-methyl-2-[(n...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC2CCCCC2C[C@H]1C(=O)NC(C)(C)C Show InChI InChI=1S/C40H54N4O4/c1-26(2)36(42-37(46)31-20-19-28-15-9-10-16-29(28)22-31)39(48)41-33(21-27-13-7-6-8-14-27)35(45)25-44-24-32-18-12-11-17-30(32)23-34(44)38(47)43-40(3,4)5/h6-10,13-16,19-20,22,26,30,32-36,45H,11-12,17-18,21,23-25H2,1-5H3,(H,41,48)(H,42,46)(H,43,47)/t30?,32?,33-,34-,35+,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283146
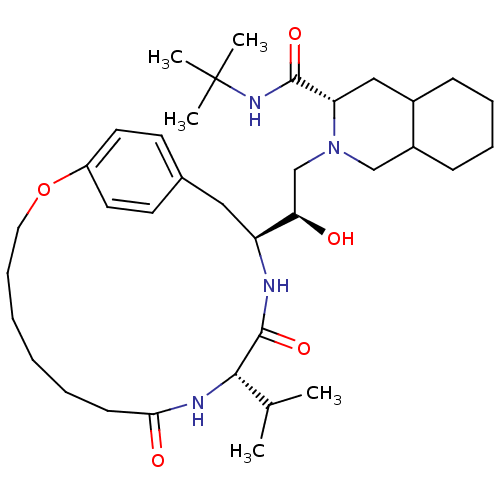
((6S,7S,8aS)-2-[(R)-2-Hydroxy-4-((S)-4-hydroxy-phen...)Show SMILES CC(C)[C@@H]1NC(=O)CCCCCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN1CC3CCCCC3C[C@H]1C(=O)NC(C)(C)C)cc2 Show InChI InChI=1S/C36H58N4O5/c1-24(2)33-35(44)37-29(20-25-15-17-28(18-16-25)45-19-11-7-6-8-14-32(42)38-33)31(41)23-40-22-27-13-10-9-12-26(27)21-30(40)34(43)39-36(3,4)5/h15-18,24,26-27,29-31,33,41H,6-14,19-23H2,1-5H3,(H,37,44)(H,38,42)(H,39,43)/t26?,27?,29-,30-,31+,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283142

((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((1S,10S)-10-isopro...)Show SMILES CC(C)[C@@H]1NC(=O)CCCCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN1CC3CCCCC3C[C@H]1C(=O)NC(C)(C)C)cc2 Show InChI InChI=1S/C35H56N4O5/c1-23(2)32-34(43)36-28(19-24-14-16-27(17-15-24)44-18-10-6-7-13-31(41)37-32)30(40)22-39-21-26-12-9-8-11-25(26)20-29(39)33(42)38-35(3,4)5/h14-17,23,25-26,28-30,32,40H,6-13,18-22H2,1-5H3,(H,36,43)(H,37,41)(H,38,42)/t25?,26?,28-,29-,30+,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283143
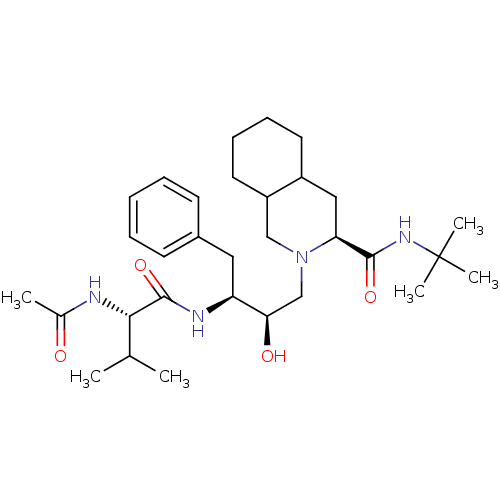
((4S,4aS,5S)-2-{(2R,3S)-3-[2-((S)-Acetylamino)-3-me...)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC2CCCCC2C[C@H]1C(=O)NC(C)(C)C Show InChI InChI=1S/C31H50N4O4/c1-20(2)28(32-21(3)36)30(39)33-25(16-22-12-8-7-9-13-22)27(37)19-35-18-24-15-11-10-14-23(24)17-26(35)29(38)34-31(4,5)6/h7-9,12-13,20,23-28,37H,10-11,14-19H2,1-6H3,(H,32,36)(H,33,39)(H,34,38)/t23?,24?,25-,26-,27+,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283147
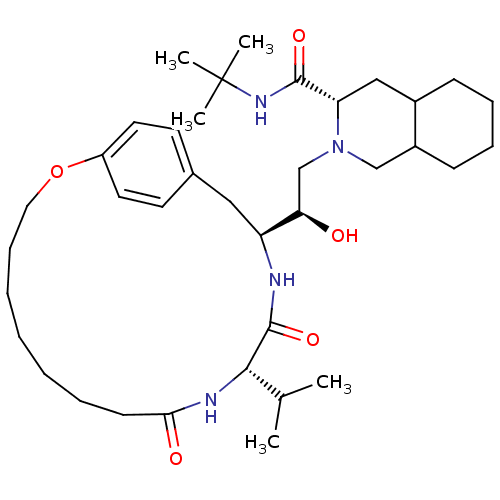
((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((1S,12S)-12-isopro...)Show SMILES CC(C)[C@@H]1NC(=O)CCCCCCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN1CC3CCCCC3C[C@H]1C(=O)NC(C)(C)C)cc2 Show InChI InChI=1S/C37H60N4O5/c1-25(2)34-36(45)38-30(21-26-16-18-29(19-17-26)46-20-12-8-6-7-9-15-33(43)39-34)32(42)24-41-23-28-14-11-10-13-27(28)22-31(41)35(44)40-37(3,4)5/h16-19,25,27-28,30-32,34,42H,6-15,20-24H2,1-5H3,(H,38,45)(H,39,43)(H,40,44)/t27?,28?,30-,31-,32+,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283154
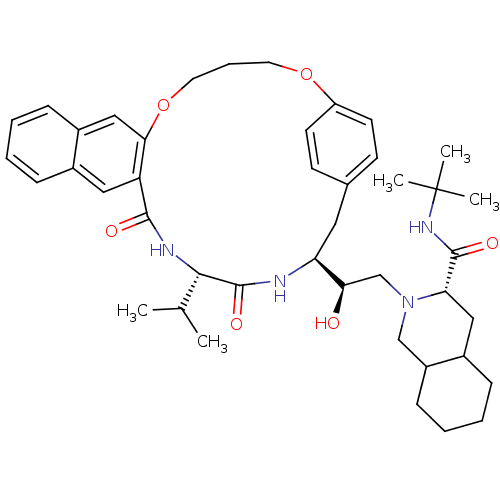
(3N-(tert-butyl)-2-{2-hydroxy-2-[19-isopropyl-17,20...)Show SMILES CC(C)[C@@H]1NC(=O)c2cc3ccccc3cc2OCCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN1CC3CCCCC3C[C@H]1C(=O)NC(C)(C)C)cc2 Show InChI InChI=1S/C43H58N4O6/c1-27(2)39-42(51)44-35(37(48)26-47-25-32-14-9-8-12-30(32)23-36(47)41(50)46-43(3,4)5)21-28-15-17-33(18-16-28)52-19-10-20-53-38-24-31-13-7-6-11-29(31)22-34(38)40(49)45-39/h6-7,11,13,15-18,22,24,27,30,32,35-37,39,48H,8-10,12,14,19-21,23,25-26H2,1-5H3,(H,44,51)(H,45,49)(H,46,50)/t30?,32?,35-,36-,37+,39-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283155
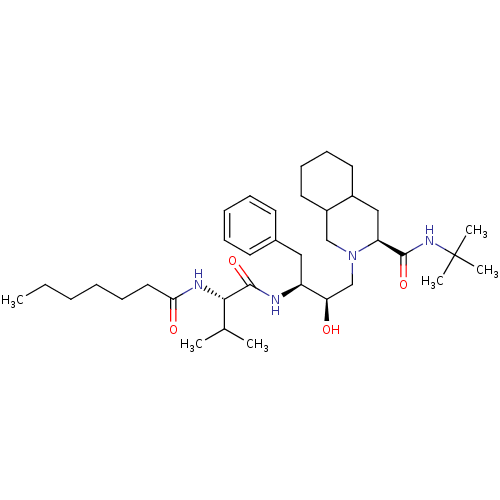
((4S,4aS,5S)-2-{(2R,3S)-3-[2-((S)-Heptanoylamino)-3...)Show SMILES CCCCCCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1CC2CCCCC2C[C@H]1C(=O)NC(C)(C)C Show InChI InChI=1S/C36H60N4O4/c1-7-8-9-13-20-32(42)38-33(25(2)3)35(44)37-29(21-26-16-11-10-12-17-26)31(41)24-40-23-28-19-15-14-18-27(28)22-30(40)34(43)39-36(4,5)6/h10-12,16-17,25,27-31,33,41H,7-9,13-15,18-24H2,1-6H3,(H,37,44)(H,38,42)(H,39,43)/t27?,28?,29-,30-,31+,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283156

((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((Z)-(1S,10S)-10-is...)Show SMILES CC(C)[C@@H]1NC(=O)\C=C/CCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN1CC3CCCCC3C[C@H]1C(=O)NC(C)(C)C)cc2 |c:7| Show InChI InChI=1S/C35H54N4O5/c1-23(2)32-34(43)36-28(19-24-14-16-27(17-15-24)44-18-10-6-7-13-31(41)37-32)30(40)22-39-21-26-12-9-8-11-25(26)20-29(39)33(42)38-35(3,4)5/h7,13-17,23,25-26,28-30,32,40H,6,8-12,18-22H2,1-5H3,(H,36,43)(H,37,41)(H,38,42)/b13-7-/t25?,26?,28-,29-,30+,32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283158

((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((1S,15S)-15-isopro...)Show SMILES CC(C)[C@@H]1NC(=O)c2ccccc2OCCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN1CC3CCCCC3C[C@H]1C(=O)NC(C)(C)C)cc2 Show InChI InChI=1S/C39H56N4O6/c1-25(2)35-38(47)40-31(33(44)24-43-23-28-12-7-6-11-27(28)22-32(43)37(46)42-39(3,4)5)21-26-15-17-29(18-16-26)48-19-10-20-49-34-14-9-8-13-30(34)36(45)41-35/h8-9,13-18,25,27-28,31-33,35,44H,6-7,10-12,19-24H2,1-5H3,(H,40,47)(H,41,45)(H,42,46)/t27?,28?,31-,32-,33+,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283148
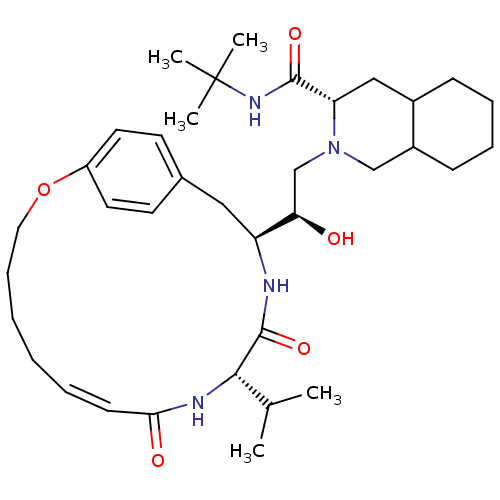
((6S,7S,8aS)-2-{(R)-2-Hydroxy-4-((S)-4-hydroxy-phen...)Show SMILES CC(C)[C@@H]1NC(=O)\C=C/CCCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN1CC3CCCCC3C[C@H]1C(=O)NC(C)(C)C)cc2 |c:7| Show InChI InChI=1S/C36H56N4O5/c1-24(2)33-35(44)37-29(20-25-15-17-28(18-16-25)45-19-11-7-6-8-14-32(42)38-33)31(41)23-40-22-27-13-10-9-12-26(27)21-30(40)34(43)39-36(3,4)5/h8,14-18,24,26-27,29-31,33,41H,6-7,9-13,19-23H2,1-5H3,(H,37,44)(H,38,42)(H,39,43)/b14-8-/t26?,27?,29-,30-,31+,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity of compound against HIV protease from BRU (IIIB) strain of HIV- 1 virus was determined |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283151

(2-[22-[2-[3-(tert-butylcarbamoyl)-(3S,4aS,8aS)-per...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC2CCCCC2CN1C[C@@H](O)[C@@H]1Cc2ccc(OCCCOc3cc4ccccc4cc3C(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2 Show InChI InChI=1S/C42H55N5O7/c1-42(2,3)46-41(52)35-21-28-10-6-7-12-30(28)24-47(35)25-36(48)33-19-26-13-15-31(16-14-26)53-17-8-18-54-37-22-29-11-5-4-9-27(29)20-32(37)39(50)45-34(23-38(43)49)40(51)44-33/h4-5,9,11,13-16,20,22,28,30,33-36,48H,6-8,10,12,17-19,21,23-25H2,1-3H3,(H2,43,49)(H,44,51)(H,45,50)(H,46,52)/t28?,30?,33-,34-,35-,36+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283157
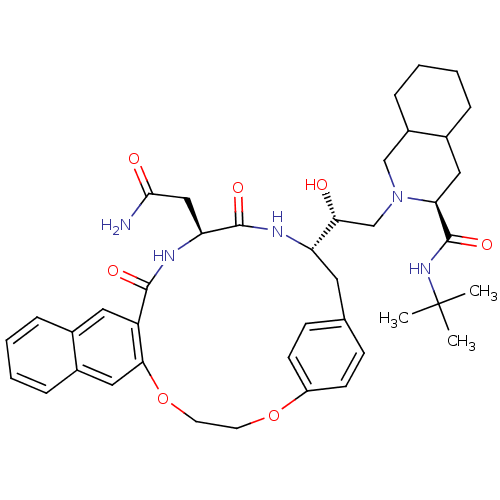
(2-[21-[2-[3-(tert-butylcarbamoyl)-(3S,4aS,8aS)-per...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC2CCCCC2CN1C[C@@H](O)[C@@H]1Cc2ccc(OCCOc3cc4ccccc4cc3C(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2 Show InChI InChI=1S/C41H53N5O7/c1-41(2,3)45-40(51)34-20-27-9-6-7-11-29(27)23-46(34)24-35(47)32-18-25-12-14-30(15-13-25)52-16-17-53-36-21-28-10-5-4-8-26(28)19-31(36)38(49)44-33(22-37(42)48)39(50)43-32/h4-5,8,10,12-15,19,21,27,29,32-35,47H,6-7,9,11,16-18,20,22-24H2,1-3H3,(H2,42,48)(H,43,50)(H,44,49)(H,45,51)/t27?,29?,32-,33-,34-,35+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283144
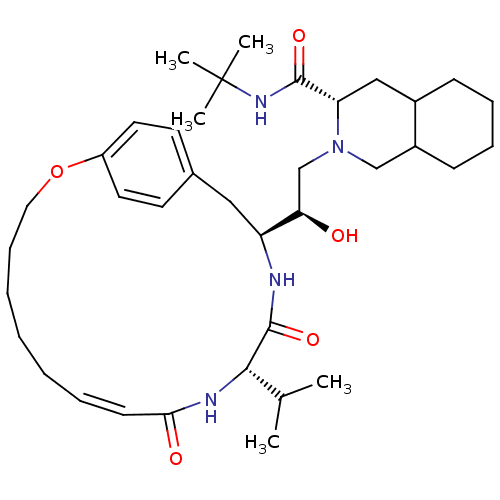
((6S,7S,8aS)-2-[(R)-2-Hydroxy-2-((Z)-(1S,12S)-12-is...)Show SMILES CC(C)[C@@H]1NC(=O)\C=C/CCCCCOc2ccc(C[C@H](NC1=O)[C@H](O)CN1CC3CCCCC3C[C@H]1C(=O)NC(C)(C)C)cc2 |c:7| Show InChI InChI=1S/C37H58N4O5/c1-25(2)34-36(45)38-30(21-26-16-18-29(19-17-26)46-20-12-8-6-7-9-15-33(43)39-34)32(42)24-41-23-28-14-11-10-13-27(28)22-31(41)35(44)40-37(3,4)5/h9,15-19,25,27-28,30-32,34,42H,6-8,10-14,20-24H2,1-5H3,(H,38,45)(H,39,43)(H,40,44)/b15-9-/t27?,28?,30-,31-,32+,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283153
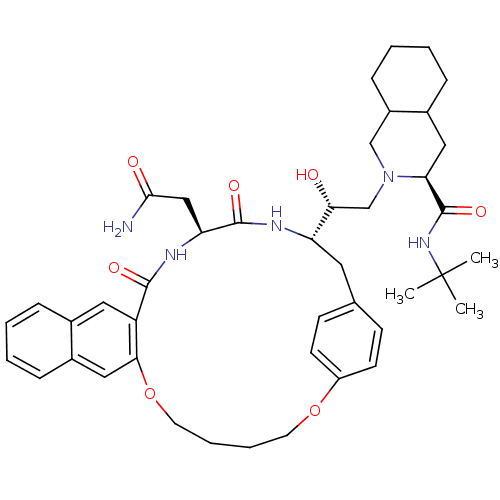
(2-[23-[2-[3-(tert-butylcarbamoyl)-(3S,4aS,8aS)-per...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CC2CCCCC2CN1C[C@@H](O)[C@@H]1Cc2ccc(OCCCCOc3cc4ccccc4cc3C(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2 Show InChI InChI=1S/C43H57N5O7/c1-43(2,3)47-42(53)36-22-29-11-6-7-13-31(29)25-48(36)26-37(49)34-20-27-14-16-32(17-15-27)54-18-8-9-19-55-38-23-30-12-5-4-10-28(30)21-33(38)40(51)46-35(24-39(44)50)41(52)45-34/h4-5,10,12,14-17,21,23,29,31,34-37,49H,6-9,11,13,18-20,22,24-26H2,1-3H3,(H2,44,50)(H,45,52)(H,46,51)(H,47,53)/t29?,31?,34-,35-,36-,37+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283141
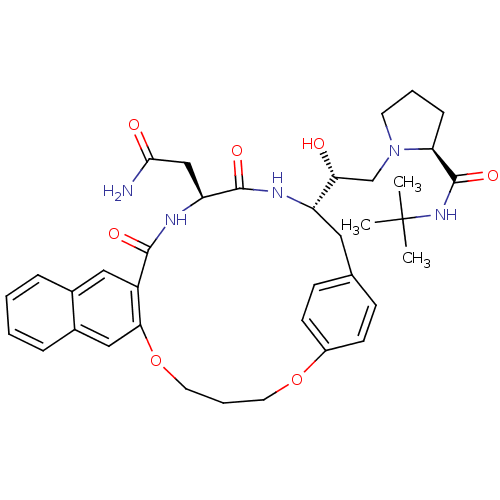
(2N-(tert-butyl)-1-{2-[19-carbamoylmethyl-17,20-dio...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CCCN1C[C@@H](O)[C@@H]1Cc2ccc(OCCCOc3cc4ccccc4cc3C(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2 Show InChI InChI=1S/C37H47N5O7/c1-37(2,3)41-36(47)30-10-6-15-42(30)22-31(43)28-18-23-11-13-26(14-12-23)48-16-7-17-49-32-20-25-9-5-4-8-24(25)19-27(32)34(45)40-29(21-33(38)44)35(46)39-28/h4-5,8-9,11-14,19-20,28-31,43H,6-7,10,15-18,21-22H2,1-3H3,(H2,38,44)(H,39,46)(H,40,45)(H,41,47)/t28-,29-,30-,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283152
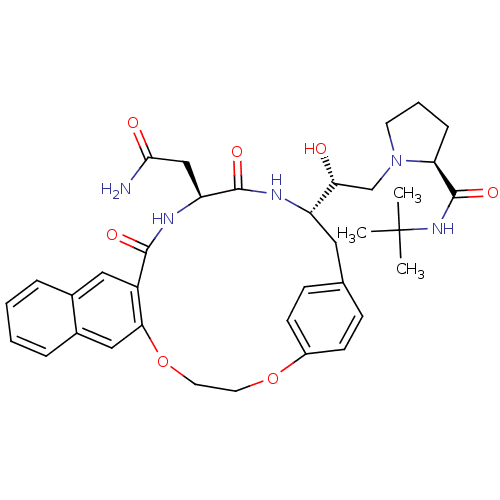
(2N-(tert-butyl)-1-{2-[18-carbamoylmethyl-16,19-dio...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CCCN1C[C@@H](O)[C@@H]1Cc2ccc(OCCOc3cc4ccccc4cc3C(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2 Show InChI InChI=1S/C36H45N5O7/c1-36(2,3)40-35(46)29-9-6-14-41(29)21-30(42)27-17-22-10-12-25(13-11-22)47-15-16-48-31-19-24-8-5-4-7-23(24)18-26(31)33(44)39-28(20-32(37)43)34(45)38-27/h4-5,7-8,10-13,18-19,27-30,42H,6,9,14-17,20-21H2,1-3H3,(H2,37,43)(H,38,45)(H,39,44)(H,40,46)/t27-,28-,29-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283149
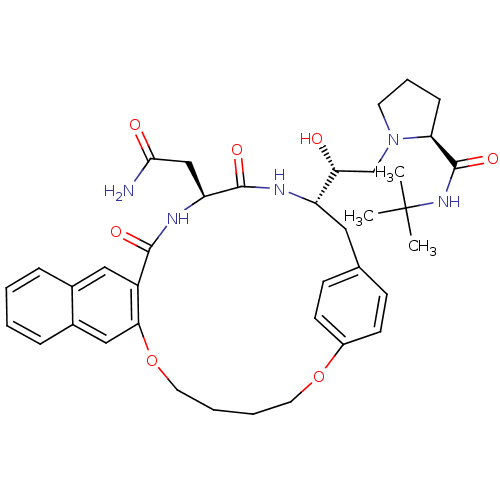
(2N-(tert-butyl)-1-{2-[20-carbamoylmethyl-18,21-dio...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CCCN1C[C@@H](O)[C@@H]1Cc2ccc(OCCCCOc3cc4ccccc4cc3C(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2 Show InChI InChI=1S/C38H49N5O7/c1-38(2,3)42-37(48)31-11-8-16-43(31)23-32(44)29-19-24-12-14-27(15-13-24)49-17-6-7-18-50-33-21-26-10-5-4-9-25(26)20-28(33)35(46)41-30(22-34(39)45)36(47)40-29/h4-5,9-10,12-15,20-21,29-32,44H,6-8,11,16-19,22-23H2,1-3H3,(H2,39,45)(H,40,47)(H,41,46)(H,42,48)/t29-,30-,31-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50283150
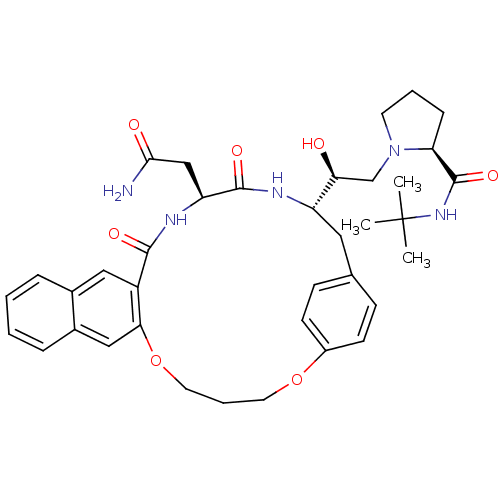
(2N-(tert-butyl)-1-{2-[19-carbamoylmethyl-17,20-dio...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CCCN1C[C@H](O)[C@@H]1Cc2ccc(OCCCOc3cc4ccccc4cc3C(=O)N[C@@H](CC(N)=O)C(=O)N1)cc2 Show InChI InChI=1S/C37H47N5O7/c1-37(2,3)41-36(47)30-10-6-15-42(30)22-31(43)28-18-23-11-13-26(14-12-23)48-16-7-17-49-32-20-25-9-5-4-8-24(25)19-27(32)34(45)40-29(21-33(38)44)35(46)39-28/h4-5,8-9,11-14,19-20,28-31,43H,6-7,10,15-18,21-22H2,1-3H3,(H2,38,44)(H,39,46)(H,40,45)(H,41,47)/t28-,29-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition HIV-1 IIIB protease. |
Bioorg Med Chem Lett 4: 2217-2222 (1994)
Article DOI: 10.1016/S0960-894X(00)80074-8
BindingDB Entry DOI: 10.7270/Q2PG1RP3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data