

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase OXA-10 (Pseudomonas aeruginosa) | BDBM50293713 (CHEMBL553476 | Tribenzyl 2-(2-phenoxyacetamido)ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Competitive inhibition of Pseudomonas aeruginosa OXA10 beta-lactamase at pH 7 | Bioorg Med Chem Lett 19: 3593-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.149 BindingDB Entry DOI: 10.7270/Q2W9597J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase OXA-10 (Pseudomonas aeruginosa) | BDBM50293712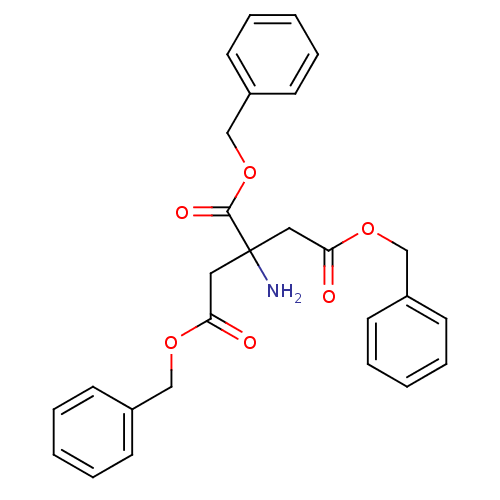 (CHEMBL561821 | Tribenzyl 2-aminopropane-1,2,3-tric...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inactivation of Pseudomonas aeruginosa OXA10 beta-lactamase at pH 7 | Bioorg Med Chem Lett 19: 3593-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.149 BindingDB Entry DOI: 10.7270/Q2W9597J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50244091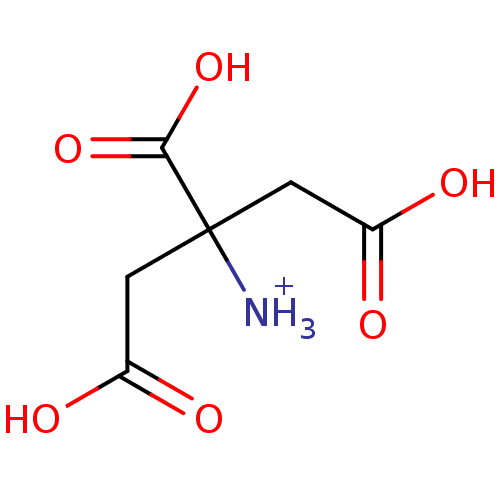 (1,2-Dicarboxy-1-carboxymethyl-ethyl-ammonium chlor...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Competitive inhibition of Bacillus licheniformis BS3 beta-lactamase at pH 5 | Bioorg Med Chem Lett 19: 3593-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.149 BindingDB Entry DOI: 10.7270/Q2W9597J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM14672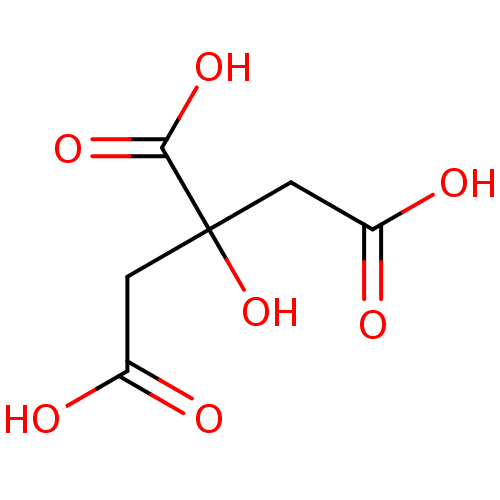 (2-hydroxypropane-1,2,3-tricarboxylic acid | CHEMBL...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of Bacillus licheniformis BS3 beta-lactamase at pH 5 | Bioorg Med Chem Lett 19: 3593-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.149 BindingDB Entry DOI: 10.7270/Q2W9597J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-lactamase (Bacillus licheniformis) | BDBM50244093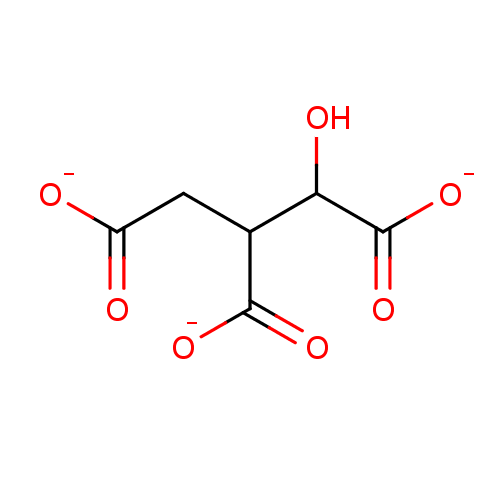 (1-hydroxypropane-1,2,3-tricarboxylate | isocitrate...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Universit£ Catholique de Louvain Curated by ChEMBL | Assay Description Inhibition of Bacillus licheniformis BS3 beta-lactamase at pH 5 | Bioorg Med Chem Lett 19: 3593-7 (2009) Article DOI: 10.1016/j.bmcl.2009.04.149 BindingDB Entry DOI: 10.7270/Q2W9597J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||