

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195819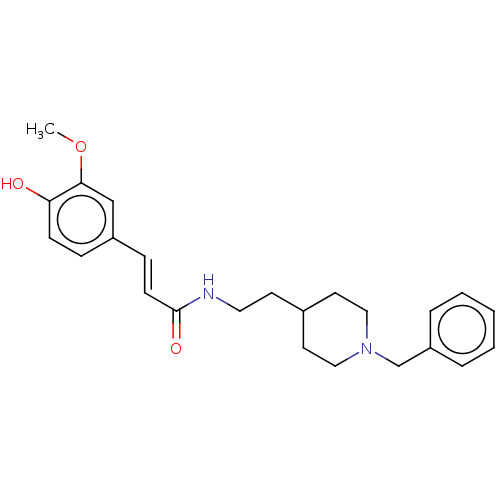 (CHEMBL3818089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195812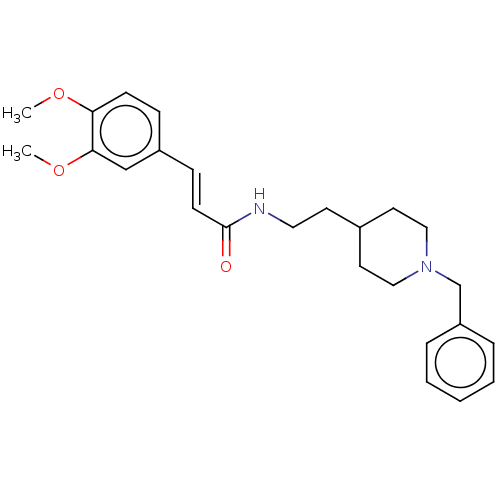 (CHEMBL3818689) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195799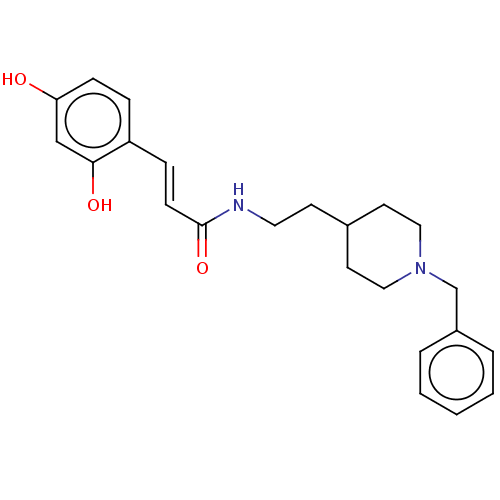 (CHEMBL3965783) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195819 (CHEMBL3818089) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195813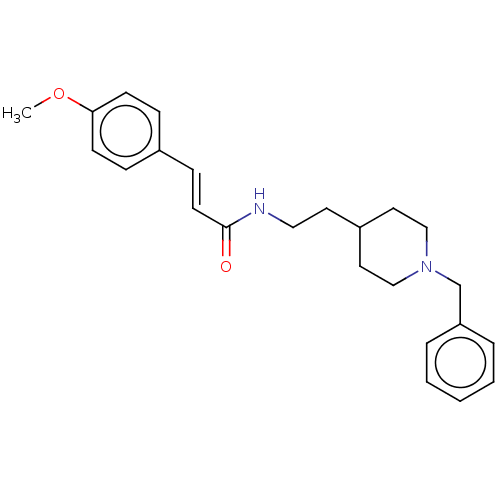 (CHEMBL3905695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195818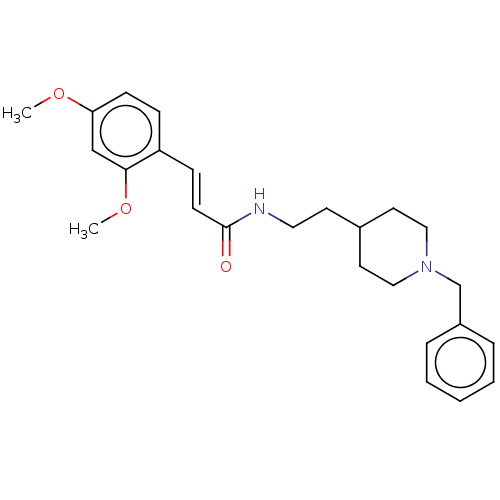 (CHEMBL3916769) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195814 (CHEMBL3818992) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195812 (CHEMBL3818689) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195855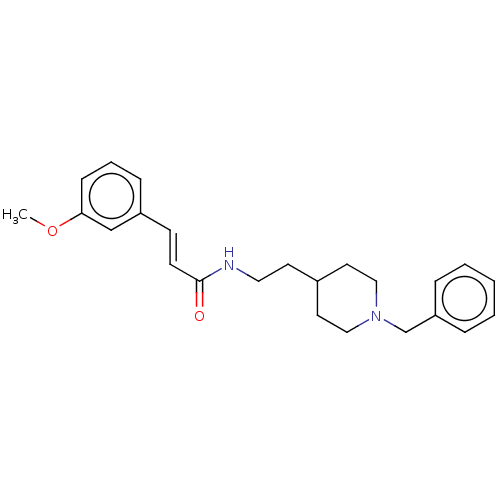 (CHEMBL3818512) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195813 (CHEMBL3905695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195836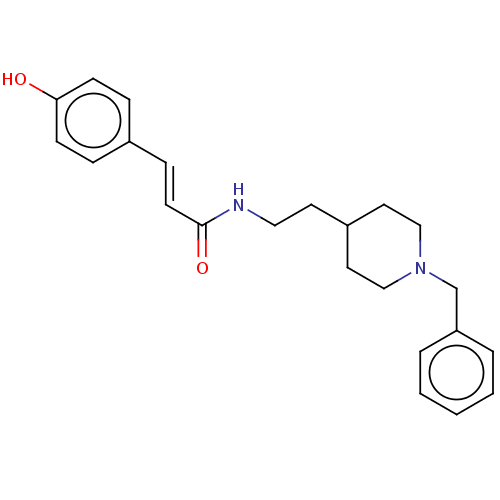 (CHEMBL3819320) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195855 (CHEMBL3818512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195799 (CHEMBL3965783) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195836 (CHEMBL3819320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195820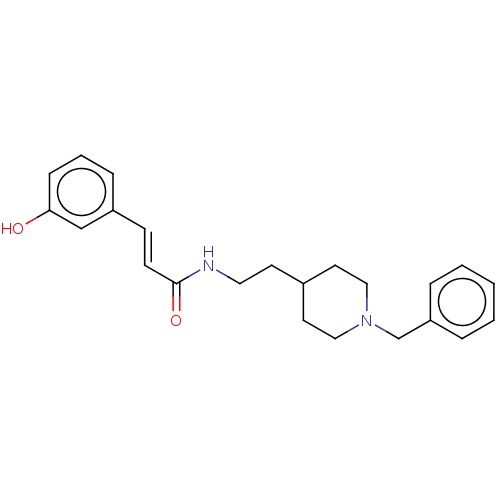 (CHEMBL3892044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195820 (CHEMBL3892044) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195814 (CHEMBL3818992) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195817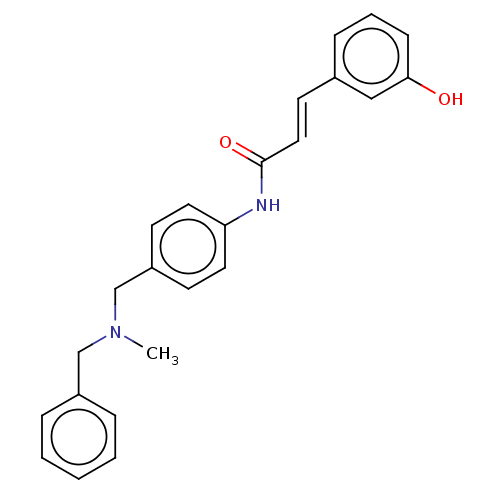 (CHEMBL3949439) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195814 (CHEMBL3818992) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195815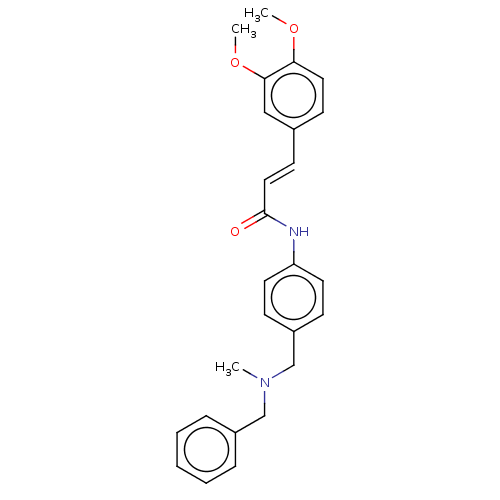 (CHEMBL3917990) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195818 (CHEMBL3916769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195811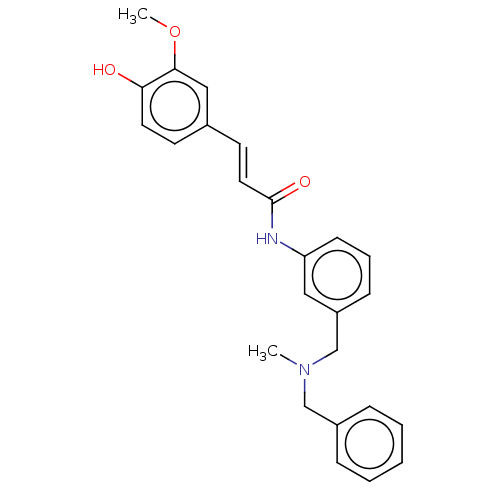 (CHEMBL3945872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195821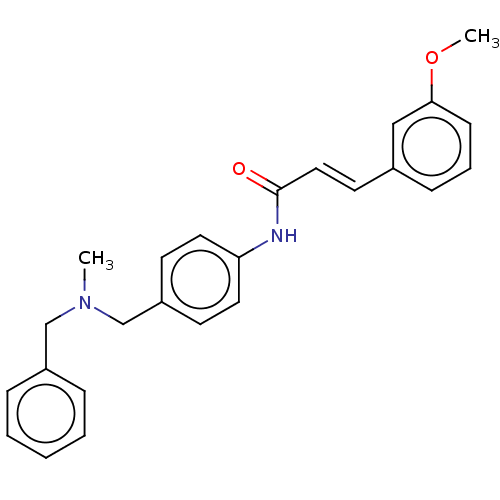 (CHEMBL3919086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195799 (CHEMBL3965783) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195870 (CHEMBL3978533) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195811 (CHEMBL3945872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195811 (CHEMBL3945872) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50195814 (CHEMBL3818992) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM29136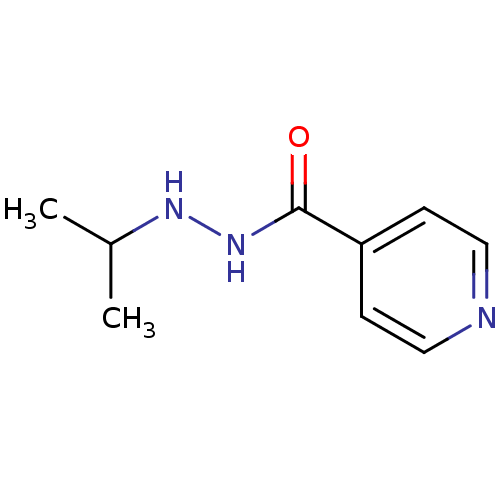 (CHEMBL92401 | Euphozid | Iprazid | Iproniazid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM29136 (CHEMBL92401 | Euphozid | Iprazid | Iproniazid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50195811 (CHEMBL3945872) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50195799 (CHEMBL3965783) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50195810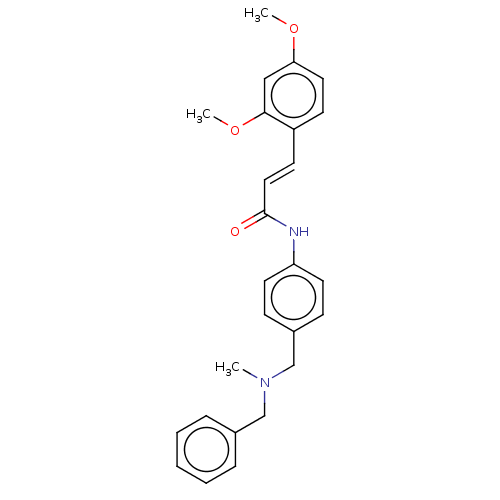 (CHEMBL3936989) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured for ... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195810 (CHEMBL3936989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50195815 (CHEMBL3917990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human serum BuChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured for 5 mins by E... | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195819 (CHEMBL3818089) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50195819 (CHEMBL3818089) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50195816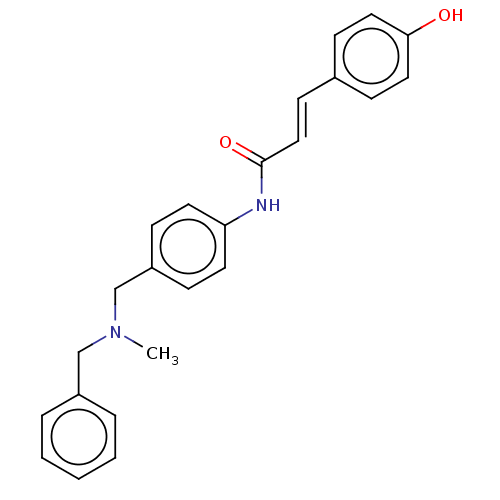 (CHEMBL3970226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195816 (CHEMBL3970226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195836 (CHEMBL3819320) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195810 (CHEMBL3936989) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50195855 (CHEMBL3818512) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOB using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195818 (CHEMBL3916769) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195870 (CHEMBL3978533) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195821 (CHEMBL3919086) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50195812 (CHEMBL3818689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA using p-tyramine as substrate incubated for 15 mins by fluorimetric method | Eur J Med Chem 121: 376-386 (2016) Article DOI: 10.1016/j.ejmech.2016.05.055 BindingDB Entry DOI: 10.7270/Q2HX1FNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |