

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of horse serum BChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of electric eel AChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50335636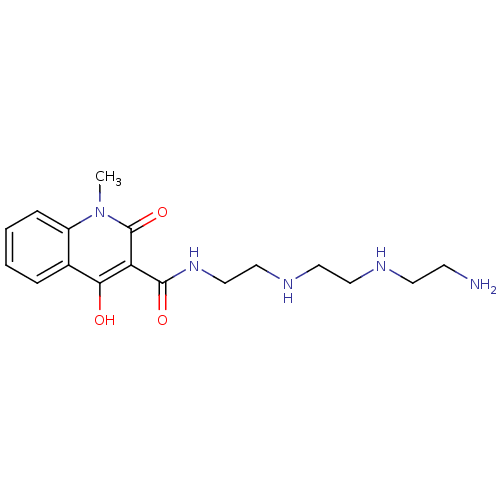 (CHEMBL1651811 | N-[2-({2-[(2-Aminoethyl)amino]ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of horse serum BChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50335633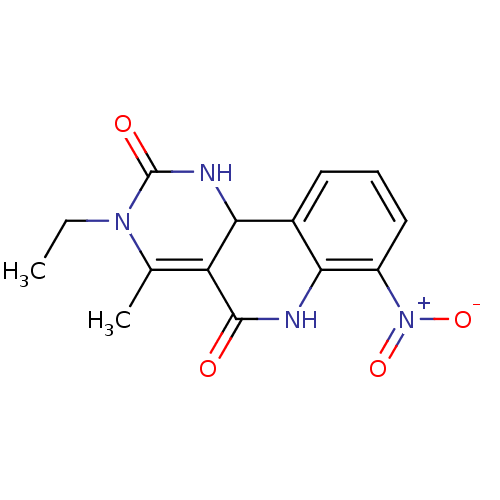 (CHEMBL1651814 | rac-3-Ethyl-4-methyl-7-nitro-6,10b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of electric eel AChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50335634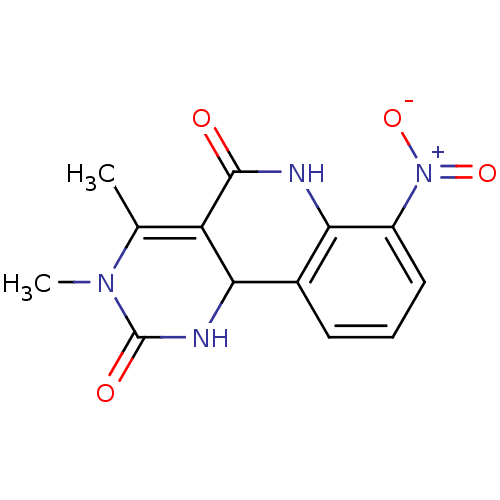 (CHEMBL1651813 | rac-3,4-Dimethyl-7-nitro-6,10b-dih...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of electric eel AChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50335635 (5-Chloro-N'-[(E)-(2,4-dihydroxyphenyl)methylidene]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of electric eel AChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50335637 (CHEMBL1651810 | N-[2-({2-[(2-Aminoethyl)amino]ethy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of electric eel AChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50335637 (CHEMBL1651810 | N-[2-({2-[(2-Aminoethyl)amino]ethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of horse serum BChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50335634 (CHEMBL1651813 | rac-3,4-Dimethyl-7-nitro-6,10b-dih...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of horse serum BChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50335633 (CHEMBL1651814 | rac-3-Ethyl-4-methyl-7-nitro-6,10b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of horse serum BChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50335636 (CHEMBL1651811 | N-[2-({2-[(2-Aminoethyl)amino]ethy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of electric eel AChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50335635 (5-Chloro-N'-[(E)-(2,4-dihydroxyphenyl)methylidene]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Franche-Comté Curated by ChEMBL | Assay Description Inhibition of horse serum BChE after 15 mins by Ellman's method | Eur J Med Chem 46: 1-10 (2010) Article DOI: 10.1016/j.ejmech.2010.08.054 BindingDB Entry DOI: 10.7270/Q2NZ87X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||