

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50344892 (CHEMBL1780187 | ethyl 2-(4-(3-(quinolin-6-yloxy)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297888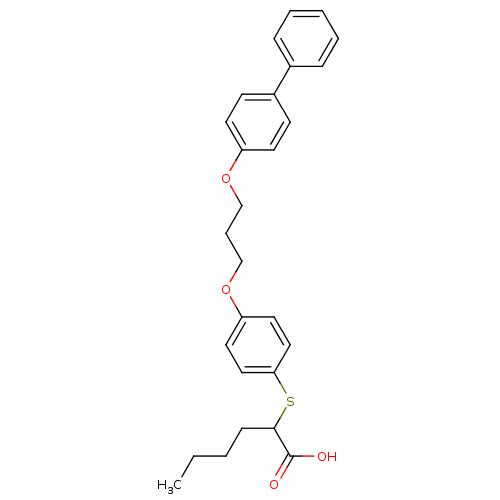 (2-(4-(3-(biphenyl-4-yloxy)propoxy)phenylthio)hexan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297879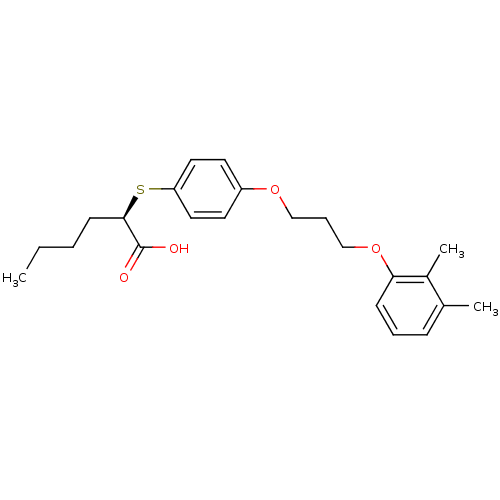 ((R)-2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297881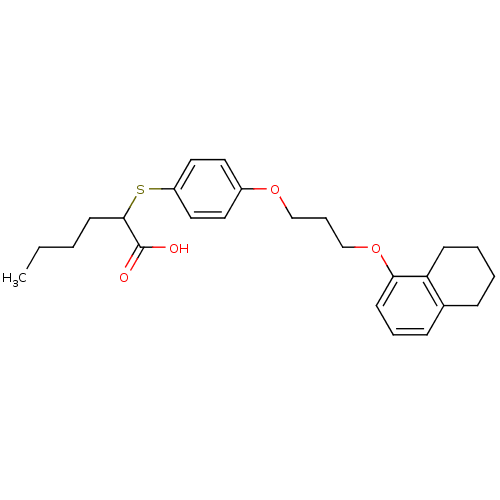 (2-(4-(3-(5,6,7,8-tetrahydronaphthalen-1-yloxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297885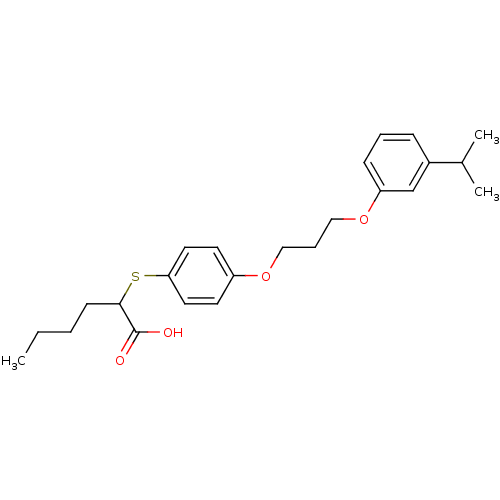 (2-(4-(3-(3-isopropylphenoxy)propoxy)phenylthio)hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297887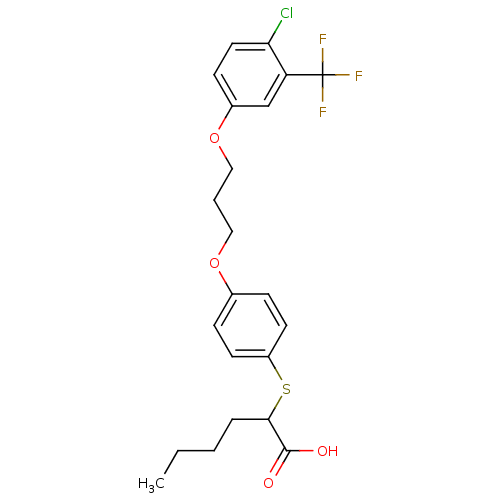 (2-(4-(3-(4-chloro-3-(trifluoromethyl)phenoxy)propo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297884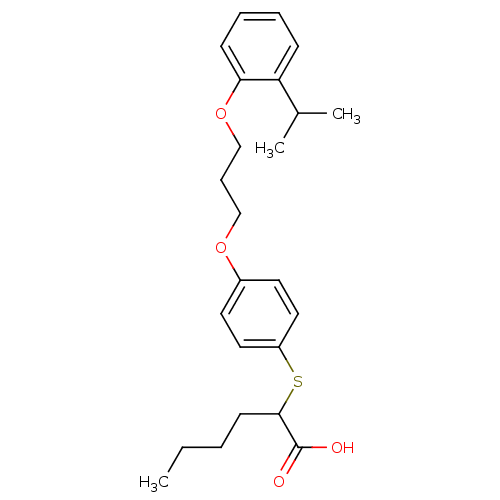 (2-(4-(3-(2-isopropylphenoxy)propoxy)phenylthio)hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50344896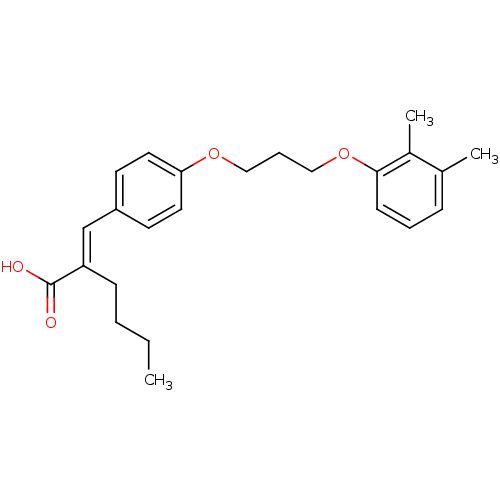 (2-(4-(3-(2,3-dimethylphenoxy)propoxy)benzylidene)h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297886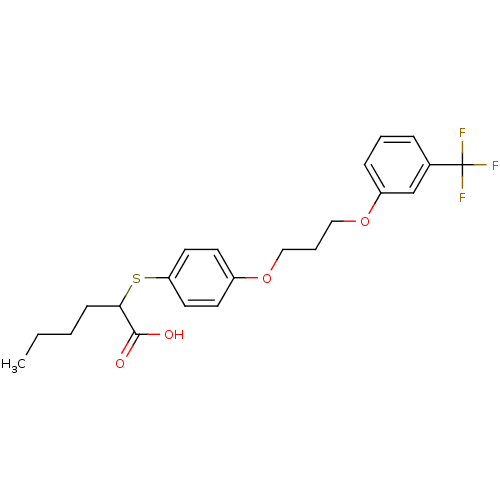 (2-(4-(3-(3-(trifluoromethyl)phenoxy)propoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297887 (2-(4-(3-(4-chloro-3-(trifluoromethyl)phenoxy)propo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297892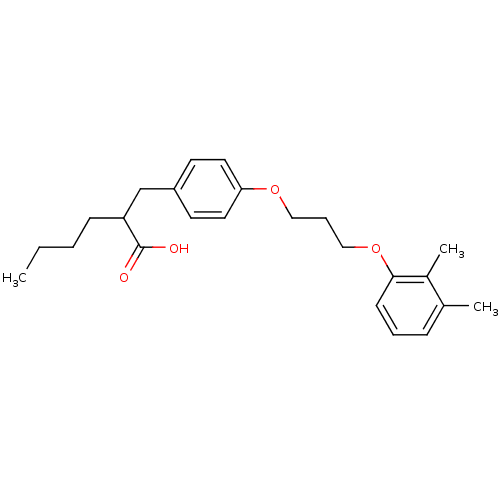 (2-(4-(3-(2,3-dimethylphenoxy)propoxy)benzyl)hexano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297887 (2-(4-(3-(4-chloro-3-(trifluoromethyl)phenoxy)propo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297880 ((S)-2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297886 (2-(4-(3-(3-(trifluoromethyl)phenoxy)propoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297888 (2-(4-(3-(biphenyl-4-yloxy)propoxy)phenylthio)hexan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297881 (2-(4-(3-(5,6,7,8-tetrahydronaphthalen-1-yloxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297885 (2-(4-(3-(3-isopropylphenoxy)propoxy)phenylthio)hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297878 (2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthio)he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297878 (2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthio)he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297888 (2-(4-(3-(biphenyl-4-yloxy)propoxy)phenylthio)hexan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297884 (2-(4-(3-(2-isopropylphenoxy)propoxy)phenylthio)hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297879 ((R)-2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297881 (2-(4-(3-(5,6,7,8-tetrahydronaphthalen-1-yloxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297880 ((S)-2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297875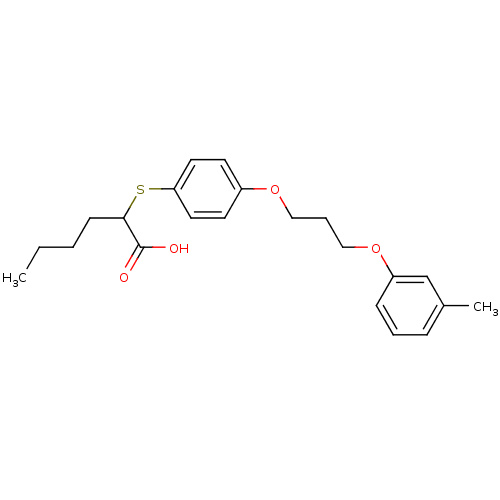 (2-(4-(3-(m-tolyloxy)propoxy)phenylthio)hexanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297874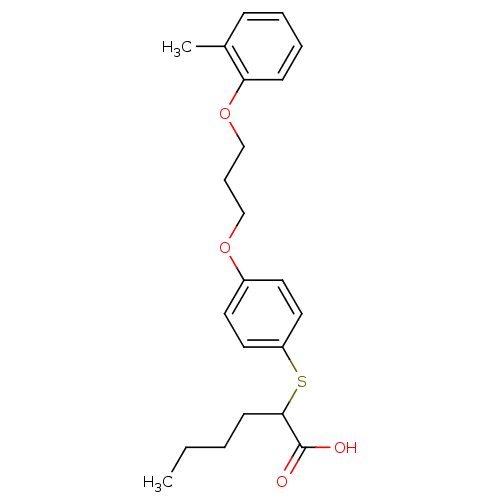 (2-(4-(3-(o-tolyloxy)propoxy)phenylthio)hexanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297883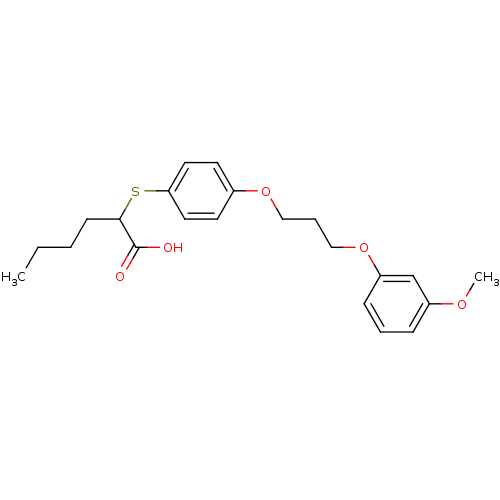 (2-(4-(3-(3-methoxyphenoxy)propoxy)phenylthio)hexan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297876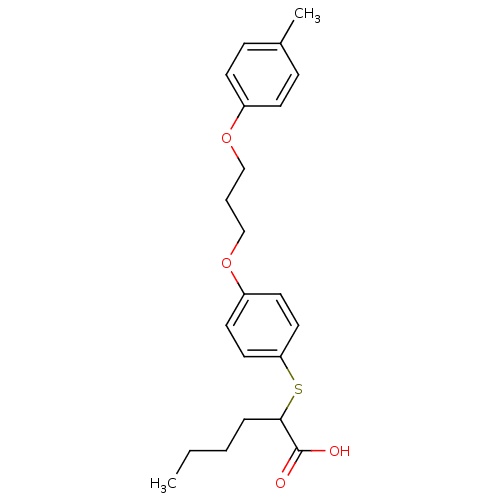 (2-(4-(3-(p-tolyloxy)propoxy)phenylthio)hexanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297885 (2-(4-(3-(3-isopropylphenoxy)propoxy)phenylthio)hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297892 (2-(4-(3-(2,3-dimethylphenoxy)propoxy)benzyl)hexano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297884 (2-(4-(3-(2-isopropylphenoxy)propoxy)phenylthio)hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297880 ((S)-2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297878 (2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthio)he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297874 (2-(4-(3-(o-tolyloxy)propoxy)phenylthio)hexanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297876 (2-(4-(3-(p-tolyloxy)propoxy)phenylthio)hexanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50344895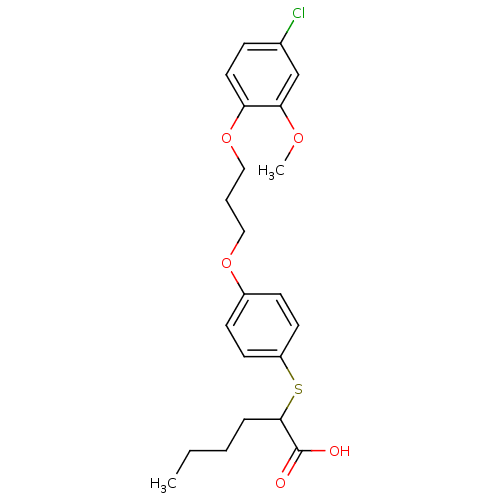 (2-(4-(3-(4-chloro-2-methoxyphenoxy)propoxy)phenylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297873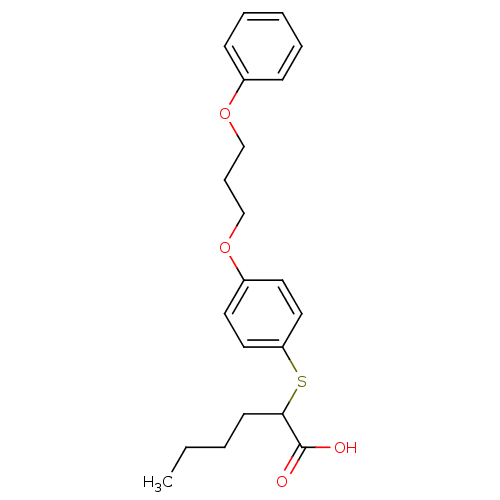 (2-(4-(3-phenoxypropoxy)-phenylthio)hexanoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in A23187-stimulated human PMNL assessed as enzyme product formation preincubated 15 mins by RP-HPLC analysis in presenc... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297886 (2-(4-(3-(3-(trifluoromethyl)phenoxy)propoxy)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297873 (2-(4-(3-phenoxypropoxy)-phenylthio)hexanoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50344896 (2-(4-(3-(2,3-dimethylphenoxy)propoxy)benzylidene)h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297891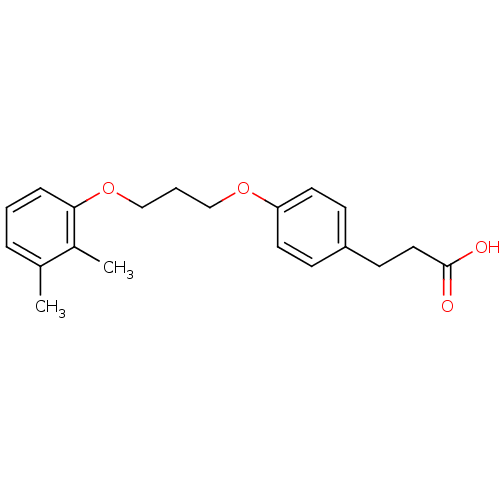 (3-(4-(3-(2,3-dimethylphenoxy)propoxy)phenyl)propan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50297890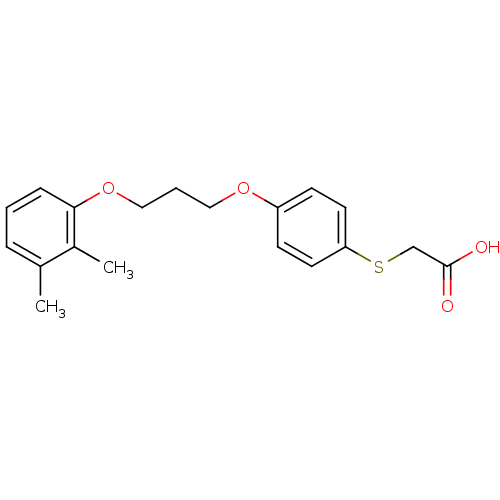 (2-(4-(3-(2,3-dimethylphenoxy)propoxy)phenylthio)ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human IL-1beta-stimulated A549 cell microsomes assessed as reduction of PGE2 formation from PGH2 after 15 mins | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50344892 (CHEMBL1780187 | ethyl 2-(4-(3-(quinolin-6-yloxy)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50297889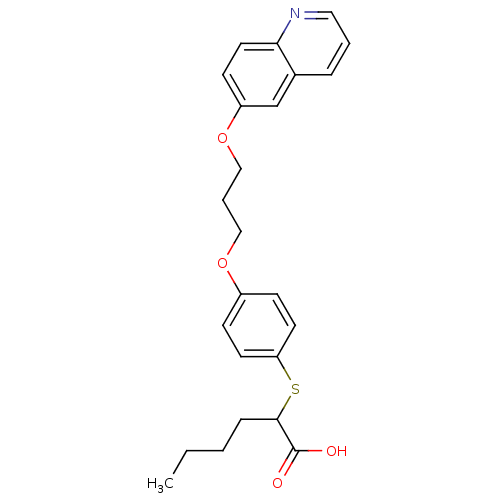 (2-(4-(3-(quinolin-6-yloxy)propoxy)phenylthio)hexan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50344893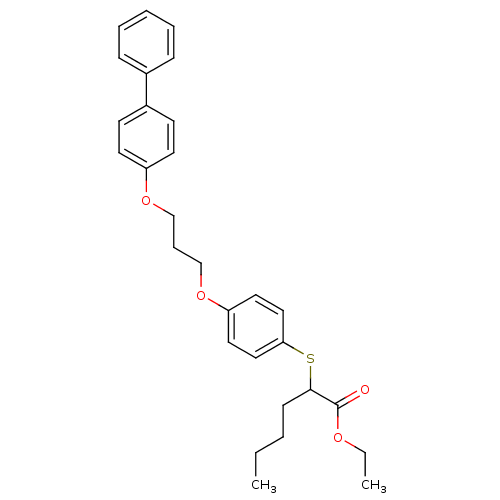 (CHEMBL1780186 | ethyl 2-(4-(3-(biphenyl-4-yloxy)pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50344894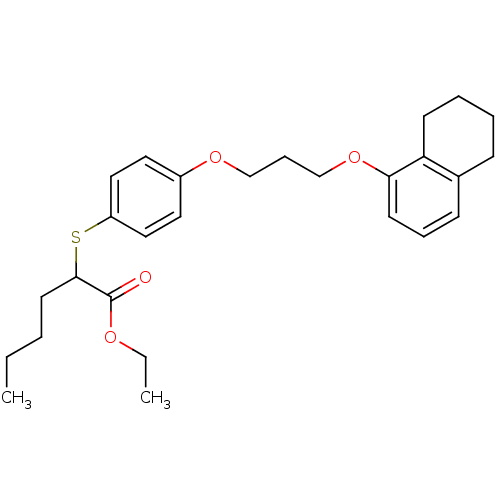 (CHEMBL1780185 | ethyl 2-(4-(3-(5,6,7,8-tetrahydron...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-lipoxygenase expressed in Escherichia coli BL21 assessed as enzyme product formation using using arachidonic acid a... | Bioorg Med Chem 19: 3394-401 (2011) Article DOI: 10.1016/j.bmc.2011.04.034 BindingDB Entry DOI: 10.7270/Q2DV1K62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 74 total ) | Next | Last >> |