 Found 94 hits Enz. Inhib. hit(s) with all data for entry = 50048871
Found 94 hits Enz. Inhib. hit(s) with all data for entry = 50048871 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229676

(CHEMBL78697)Show InChI InChI=1S/C7H12N6/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2,(H2,8,10)/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229669
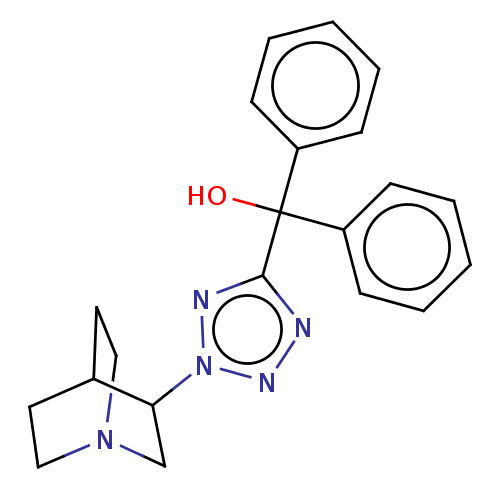
(CHEMBL309432)Show SMILES OC(c1nnn(n1)C1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(9.08,-4.44,;7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,;8.45,-3.21,;7.81,-1.81,;8.71,-.56,;10.25,-.72,;10.88,-2.13,;9.98,-3.37,;8.16,-5.85,;7.26,-7.1,;7.87,-8.5,;9.41,-8.66,;10.31,-7.41,;9.69,-6.01,)| Show InChI InChI=1S/C21H23N5O/c27-21(17-7-3-1-4-8-17,18-9-5-2-6-10-18)20-22-24-26(23-20)19-15-25-13-11-16(19)12-14-25/h1-10,16,19,27H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229684
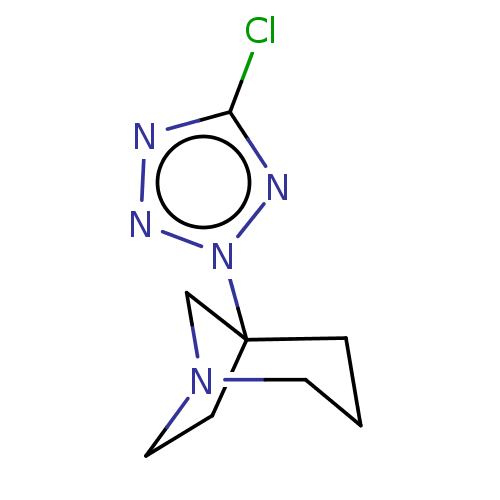
(CHEMBL78615)Show InChI InChI=1S/C8H12ClN5/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229669

(CHEMBL309432)Show SMILES OC(c1nnn(n1)C1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(9.08,-4.44,;7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,;8.45,-3.21,;7.81,-1.81,;8.71,-.56,;10.25,-.72,;10.88,-2.13,;9.98,-3.37,;8.16,-5.85,;7.26,-7.1,;7.87,-8.5,;9.41,-8.66,;10.31,-7.41,;9.69,-6.01,)| Show InChI InChI=1S/C21H23N5O/c27-21(17-7-3-1-4-8-17,18-9-5-2-6-10-18)20-22-24-26(23-20)19-15-25-13-11-16(19)12-14-25/h1-10,16,19,27H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229617
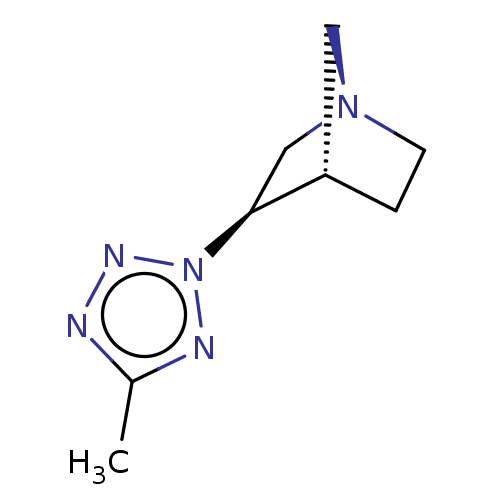
(CHEMBL311799)Show InChI InChI=1S/C8H13N5/c1-6-9-11-13(10-6)8-5-12-3-2-7(8)4-12/h7-8H,2-5H2,1H3/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229681
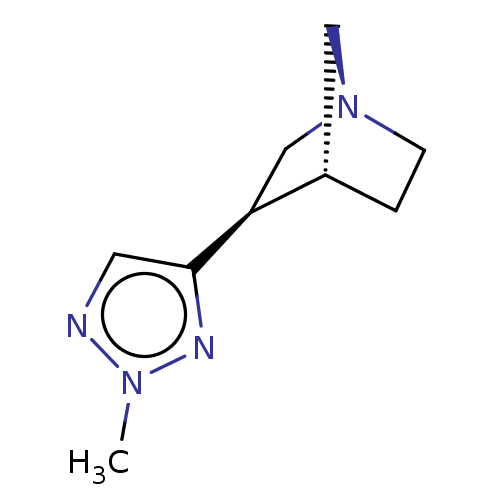
(CHEMBL79040)Show InChI InChI=1S/C9H14N4/c1-12-10-4-9(11-12)8-6-13-3-2-7(8)5-13/h4,7-8H,2-3,5-6H2,1H3/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229685
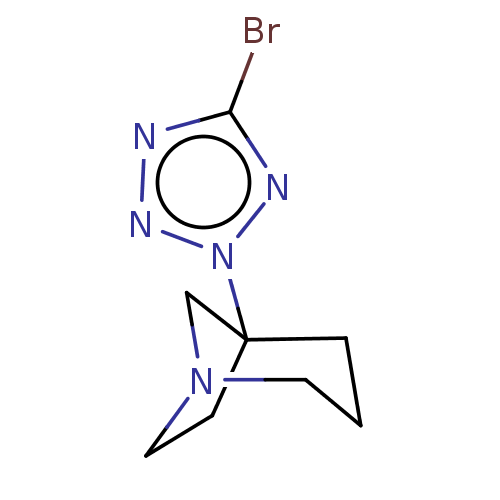
(CHEMBL311212)Show InChI InChI=1S/C8H12BrN5/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229678
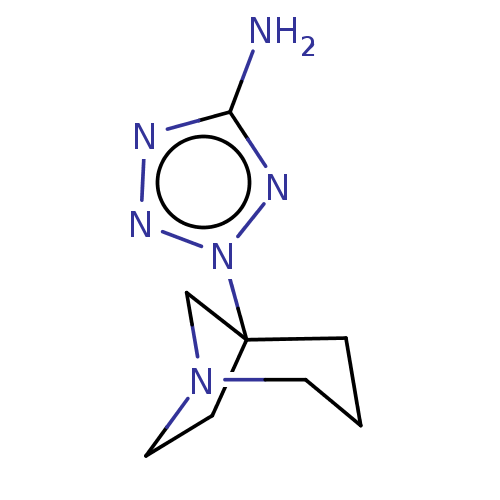
(CHEMBL80723)Show InChI InChI=1S/C8H14N6/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2,(H2,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229696

(CHEMBL419659)Show InChI InChI=1S/C7H10BrN5/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229694
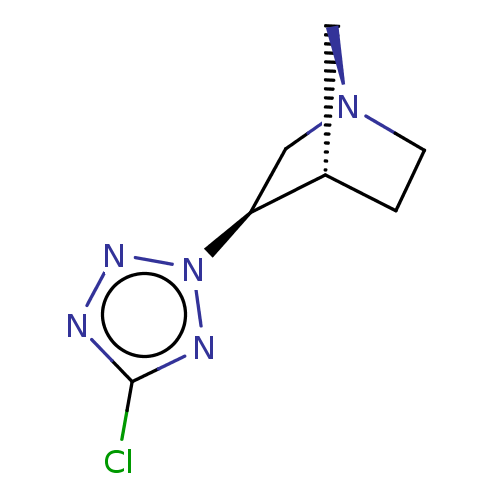
(CHEMBL81481)Show InChI InChI=1S/C7H10ClN5/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229623

(CHEMBL81281)Show InChI InChI=1S/C8H13N5/c1-12-10-8(9-11-12)7-5-13-3-2-6(7)4-13/h6-7H,2-5H2,1H3/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM10759
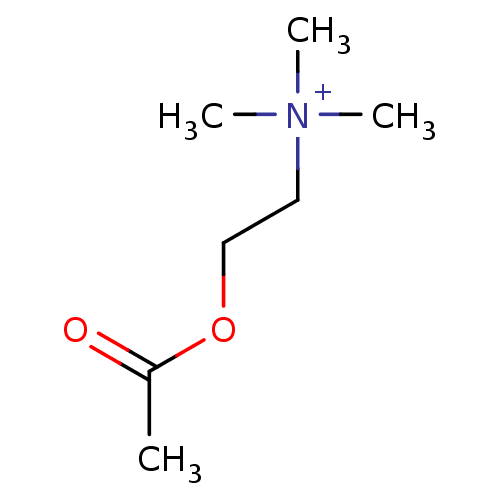
(2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...)Show InChI InChI=1S/C7H16NO2/c1-7(9)10-6-5-8(2,3)4/h5-6H2,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229692
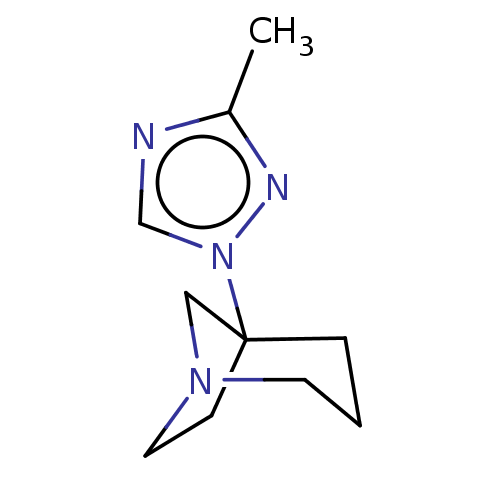
(CHEMBL311562)Show InChI InChI=1S/C10H16N4/c1-9-11-8-14(12-9)10-3-2-5-13(7-10)6-4-10/h8H,2-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229675
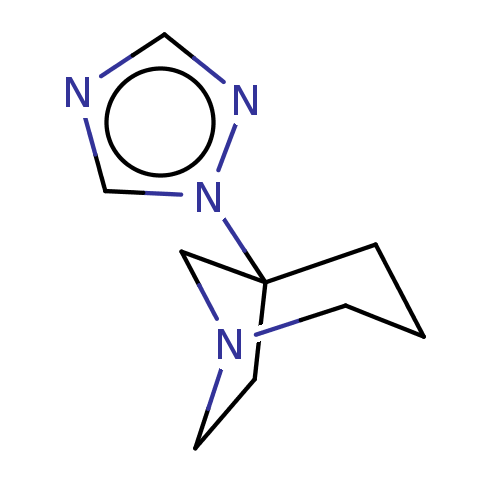
(CHEMBL309638)Show InChI InChI=1S/C9H14N4/c1-2-9(13-8-10-7-11-13)3-5-12(4-1)6-9/h7-8H,1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229672

(CHEMBL309729)Show InChI InChI=1S/C9H15N5/c10-8-11-7-14(12-8)9-2-1-4-13(6-9)5-3-9/h7H,1-6H2,(H2,10,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50005677
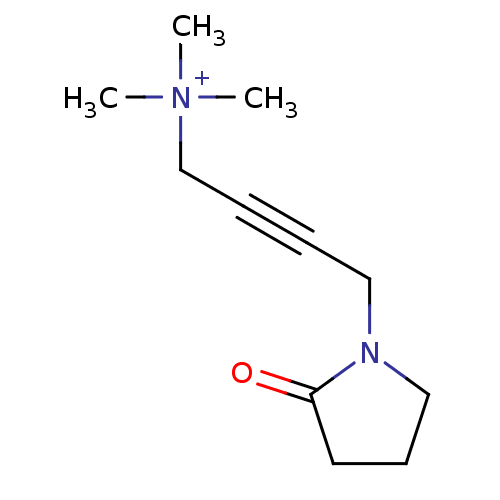
(CHEMBL23957 | OXO-M | Trimethyl-[4-(2-oxo-pyrrolid...)Show InChI InChI=1S/C11H19N2O/c1-13(2,3)10-5-4-8-12-9-6-7-11(12)14/h6-10H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229673

(CHEMBL311703)Show InChI InChI=1S/C9H15N5/c1-8-10-12-14(11-8)9-3-2-5-13(7-9)6-4-9/h2-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229693
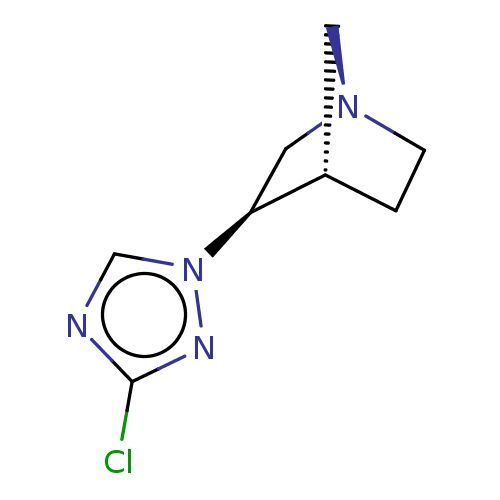
(CHEMBL312434)Show InChI InChI=1S/C8H11ClN4/c9-8-10-5-13(11-8)7-4-12-2-1-6(7)3-12/h5-7H,1-4H2/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229621
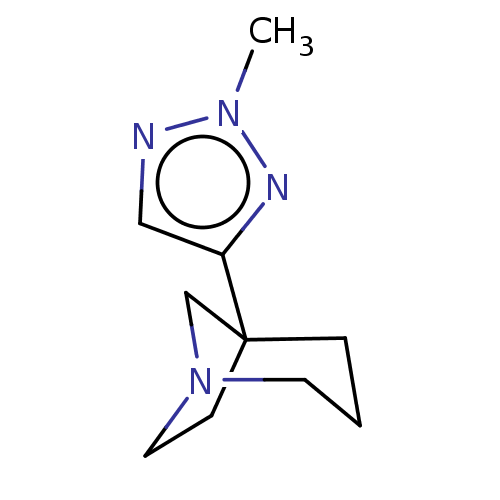
(CHEMBL311036)Show InChI InChI=1S/C10H16N4/c1-13-11-7-9(12-13)10-3-2-5-14(8-10)6-4-10/h7H,2-6,8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229683

(CHEMBL79020)Show InChI InChI=1S/C9H14N4/c1-12-10-4-9(11-12)8-6-13-3-2-7(8)5-13/h4,7-8H,2-3,5-6H2,1H3/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229698

(CHEMBL81492)Show InChI InChI=1S/C9H14N4/c1-7-10-6-13(11-7)9-5-12-3-2-8(9)4-12/h6,8-9H,2-5H2,1H3/t8-,9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229686
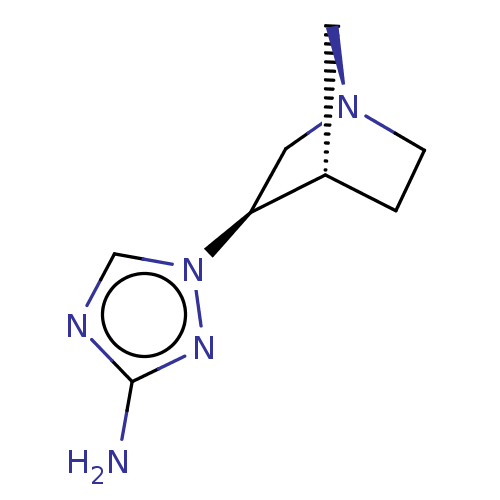
(CHEMBL311989)Show InChI InChI=1S/C8H13N5/c9-8-10-5-13(11-8)7-4-12-2-1-6(7)3-12/h5-7H,1-4H2,(H2,9,11)/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229619
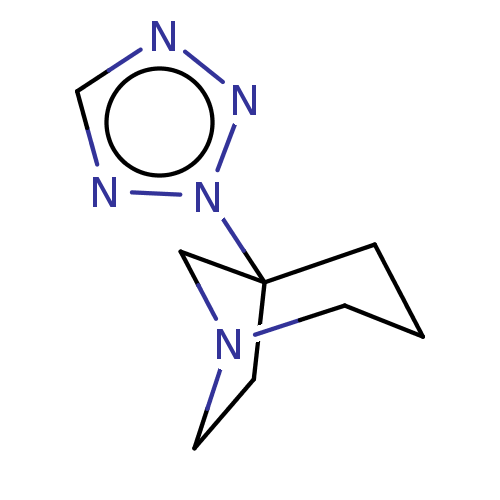
(CHEMBL442975)Show InChI InChI=1S/C8H13N5/c1-2-8(13-10-7-9-11-13)3-5-12(4-1)6-8/h7H,1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229680
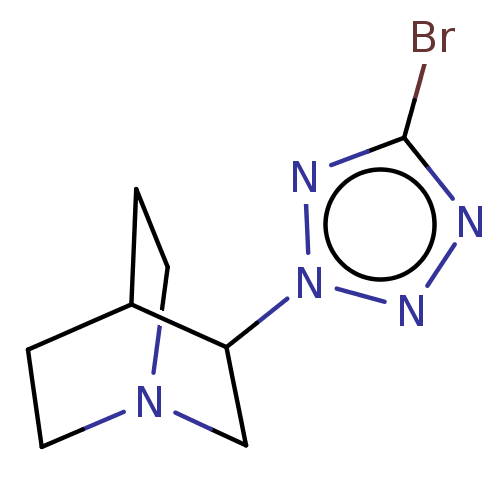
(CHEMBL312656)Show SMILES Brc1nnn(n1)C1CN2CCC1CC2 |(7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,)| Show InChI InChI=1S/C8H12BrN5/c9-8-10-12-14(11-8)7-5-13-3-1-6(7)2-4-13/h6-7H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229614

(CHEMBL78964)Show SMILES Clc1nnn(n1)C1CN2CCC1CC2 |(7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,)| Show InChI InChI=1S/C8H12ClN5/c9-8-10-12-14(11-8)7-5-13-3-1-6(7)2-4-13/h6-7H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229615
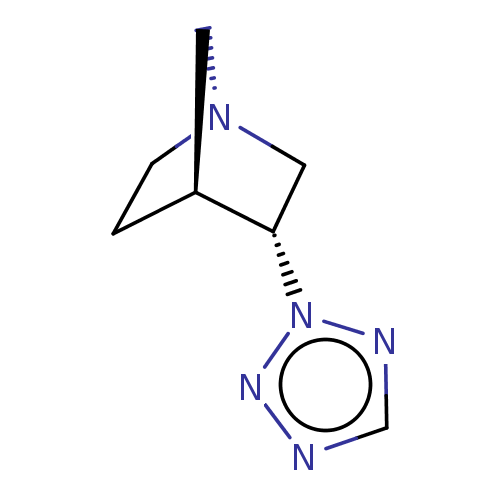
(CHEMBL81314)Show InChI InChI=1S/C7H11N5/c1-2-11-3-6(1)7(4-11)12-9-5-8-10-12/h5-7H,1-4H2/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229691
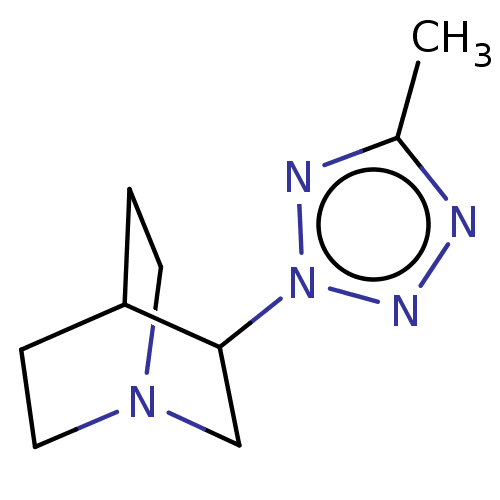
(CHEMBL23118)Show SMILES Cc1nnn(n1)C1CN2CCC1CC2 |(18.11,-12.96,;17.7,-14.41,;18.77,-15.8,;17.57,-17.13,;16.17,-16.52,;16.34,-14.95,;14.82,-17.27,;14.82,-18.82,;13.48,-19.48,;12.16,-18.82,;12.16,-17.27,;13.46,-16.5,;13.87,-17.98,;12.85,-18.33,)| Show InChI InChI=1S/C9H15N5/c1-7-10-12-14(11-7)9-6-13-4-2-8(9)3-5-13/h8-9H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229687
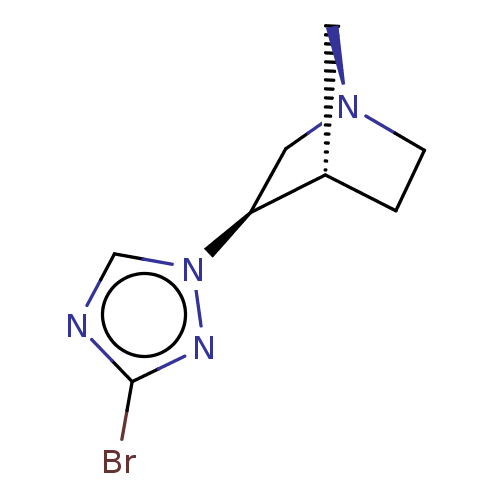
(CHEMBL308343)Show InChI InChI=1S/C8H11BrN4/c9-8-10-5-13(11-8)7-4-12-2-1-6(7)3-12/h5-7H,1-4H2/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229682
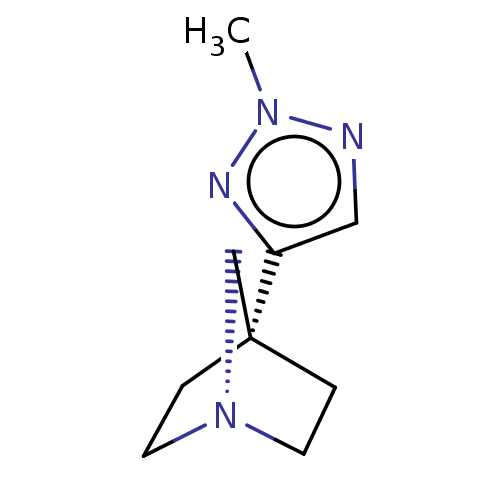
(CHEMBL79374)Show InChI InChI=1S/C9H14N4/c1-12-10-6-8(11-12)9-2-4-13(7-9)5-3-9/h6H,2-5,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229690

(CHEMBL311297)Show SMILES Nc1nnn(n1)C1CN2CCC1CC2 |(7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,)| Show InChI InChI=1S/C8H14N6/c9-8-10-12-14(11-8)7-5-13-3-1-6(7)2-4-13/h6-7H,1-5H2,(H2,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229674
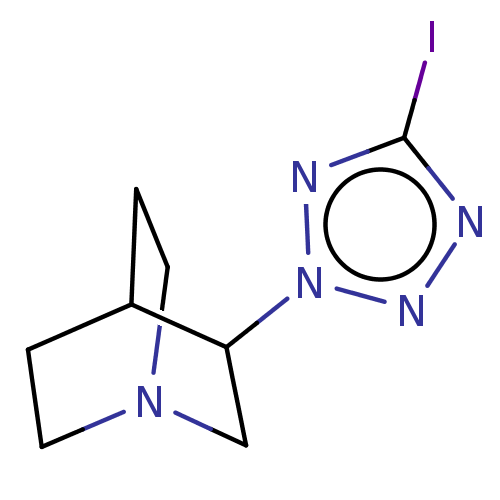
(CHEMBL81968)Show SMILES Ic1nnn(n1)C1CN2CCC1CC2 |(7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,)| Show InChI InChI=1S/C8H12IN5/c9-8-10-12-14(11-8)7-5-13-3-1-6(7)2-4-13/h6-7H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229620

(CHEMBL421332)Show InChI InChI=1S/C7H10IN5/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229697
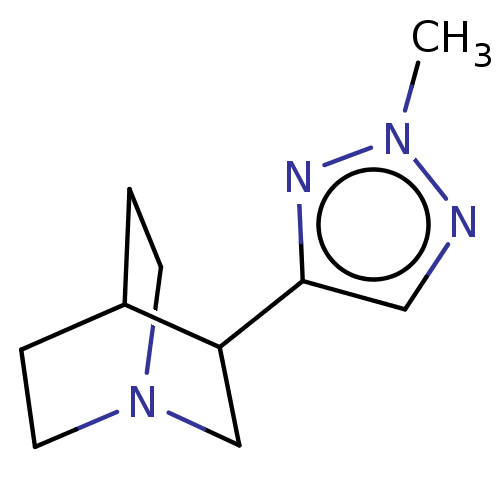
(CHEMBL23721)Show SMILES Cn1ncc(n1)C1CN2CCC1CC2 |(18.33,-12.98,;17.57,-14.14,;18.65,-15.55,;17.46,-16.88,;16.04,-16.24,;16.2,-14.7,;14.7,-17.02,;14.7,-18.55,;13.34,-19.21,;12.03,-18.55,;12.03,-17.02,;13.35,-16.24,;13.75,-17.74,;12.73,-18.07,)| Show InChI InChI=1S/C10H16N4/c1-13-11-6-10(12-13)9-7-14-4-2-8(9)3-5-14/h6,8-9H,2-5,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229618
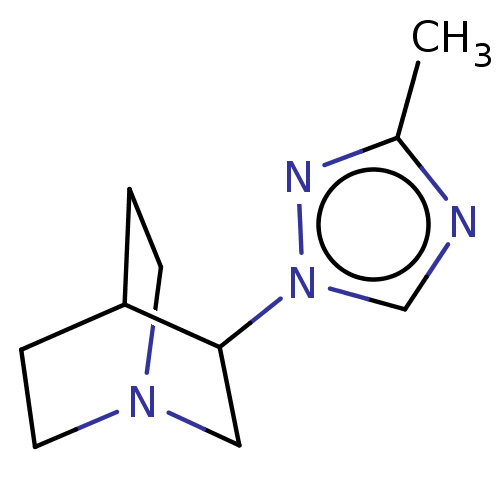
(CHEMBL23335)Show SMILES Cc1ncn(n1)C1CN2CCC1CC2 |(17.8,-12.62,;17.41,-14.06,;18.48,-15.44,;17.28,-16.78,;15.88,-16.13,;16.04,-14.6,;14.52,-16.91,;14.52,-18.45,;13.19,-19.11,;11.88,-18.45,;11.88,-16.91,;13.19,-16.13,;13.59,-17.62,;12.57,-17.96,)| Show InChI InChI=1S/C10H16N4/c1-8-11-7-14(12-8)10-6-13-4-2-9(10)3-5-13/h7,9-10H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229689
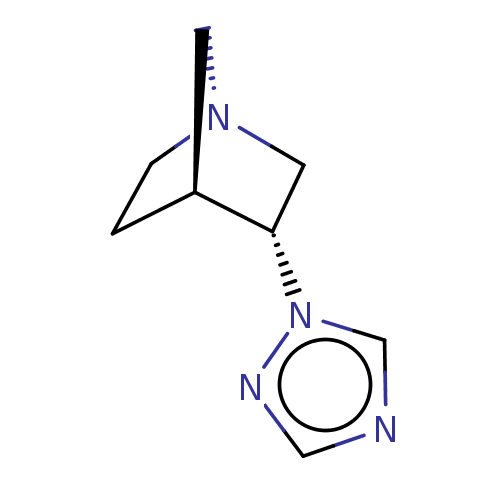
(CHEMBL309498)Show InChI InChI=1S/C8H12N4/c1-2-11-3-7(1)8(4-11)12-6-9-5-10-12/h5-8H,1-4H2/t7-,8-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229671
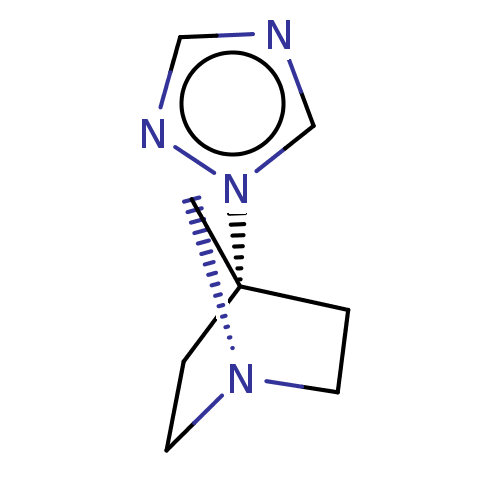
(CHEMBL311008)Show InChI InChI=1S/C8H12N4/c1-3-11-4-2-8(1,5-11)12-7-9-6-10-12/h6-7H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229679
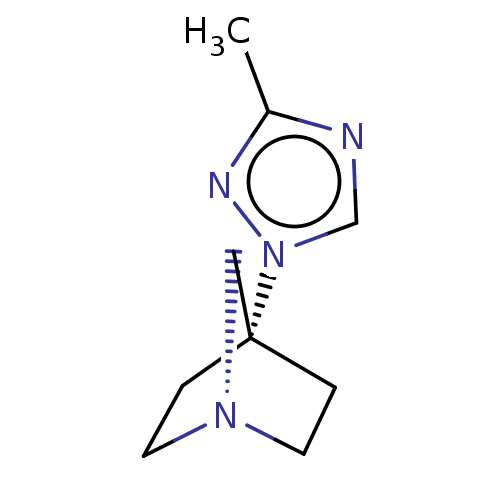
(CHEMBL280219)Show InChI InChI=1S/C9H14N4/c1-8-10-7-13(11-8)9-2-4-12(6-9)5-3-9/h7H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229695
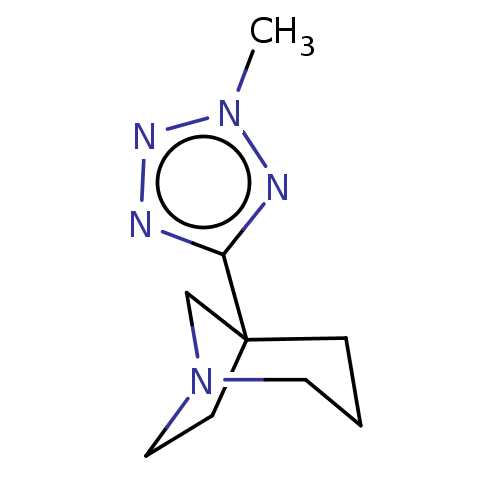
(CHEMBL309281)Show InChI InChI=1S/C9H15N5/c1-13-11-8(10-12-13)9-3-2-5-14(7-9)6-4-9/h2-7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM46858
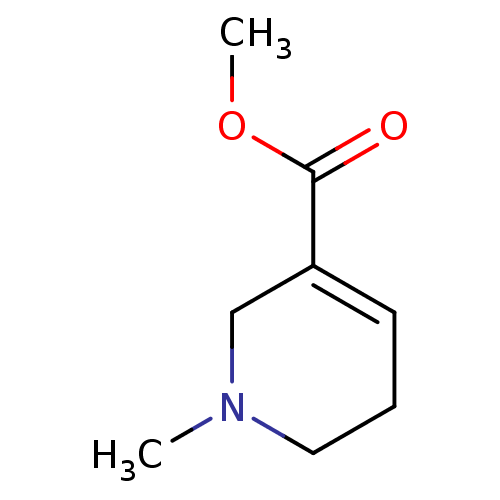
(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229688
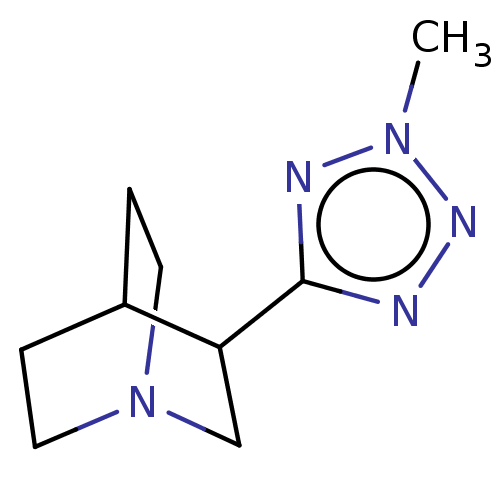
(CHEMBL276975)Show SMILES Cn1nnc(n1)C1CN2CCC1CC2 |(18.04,-12.91,;17.63,-14.36,;18.7,-15.74,;17.5,-17.06,;16.11,-16.45,;16.28,-14.89,;14.77,-17.21,;14.77,-18.75,;13.43,-19.41,;12.11,-18.75,;12.11,-17.21,;13.41,-16.43,;13.82,-17.91,;12.8,-18.26,)| Show InChI InChI=1S/C9H15N5/c1-13-11-9(10-12-13)8-6-14-4-2-7(8)3-5-14/h7-8H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM39341
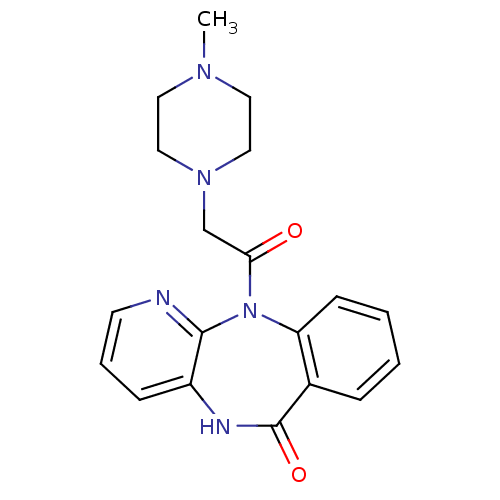
(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229674

(CHEMBL81968)Show SMILES Ic1nnn(n1)C1CN2CCC1CC2 |(7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,)| Show InChI InChI=1S/C8H12IN5/c9-8-10-12-14(11-8)7-5-13-3-1-6(7)2-4-13/h6-7H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229667
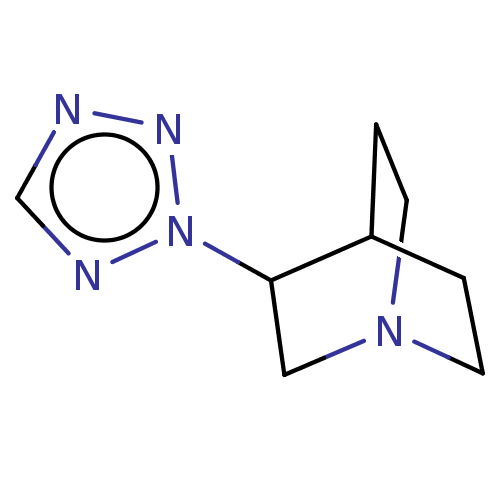
(CHEMBL23563)Show SMILES C1CN2CCC1C(C2)n1ncnn1 |(12.18,-16.59,;12.18,-18.13,;13.51,-18.81,;12.88,-17.66,;13.9,-17.31,;13.49,-15.8,;14.85,-16.59,;14.85,-18.13,;16.2,-15.82,;16.37,-14.27,;17.73,-13.71,;18.81,-15.11,;17.61,-16.47,)| Show InChI InChI=1S/C8H13N5/c1-3-12-4-2-7(1)8(5-12)13-10-6-9-11-13/h6-8H,1-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229622
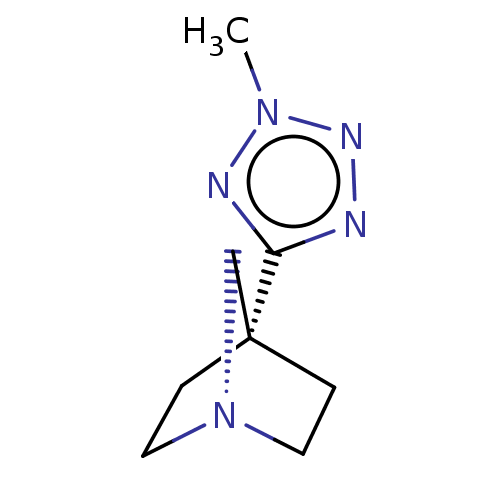
(CHEMBL311246)Show InChI InChI=1S/C8H13N5/c1-12-10-7(9-11-12)8-2-4-13(6-8)5-3-8/h2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229685

(CHEMBL311212)Show InChI InChI=1S/C8H12BrN5/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex Muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 322 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229677
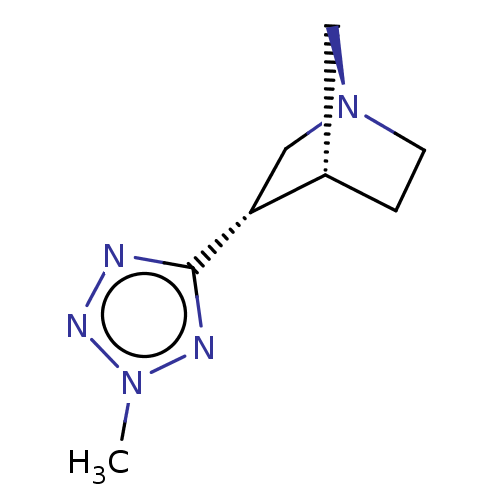
(CHEMBL79265)Show InChI InChI=1S/C8H13N5/c1-12-10-8(9-11-12)7-5-13-3-2-6(7)4-13/h6-7H,2-5H2,1H3/t6-,7+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229684

(CHEMBL78615)Show InChI InChI=1S/C8H12ClN5/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 332 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229620

(CHEMBL421332)Show InChI InChI=1S/C7H10IN5/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data