

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231010 (CHEMBL312066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231140 (CHEMBL77029) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145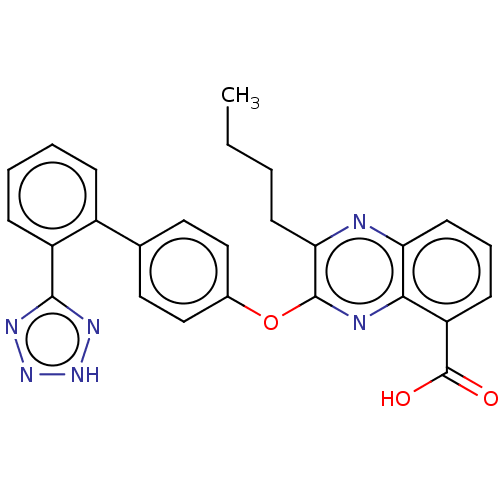 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231150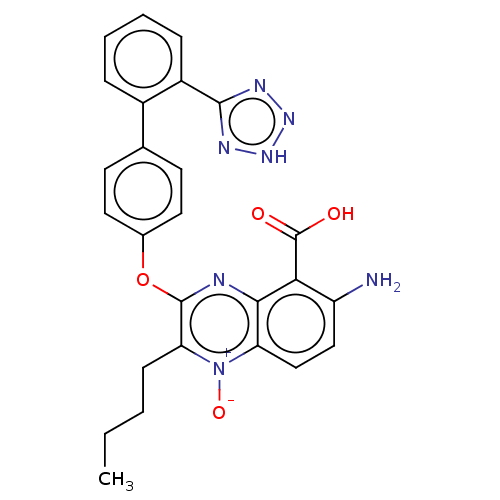 (CHEMBL77935) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231139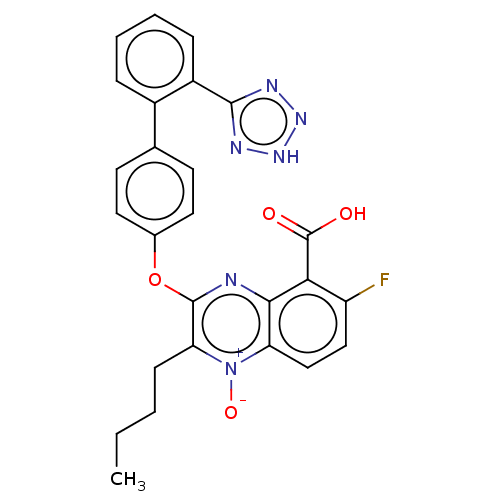 (CHEMBL77827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231149 (CHEMBL306278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231143 (CHEMBL75053) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231007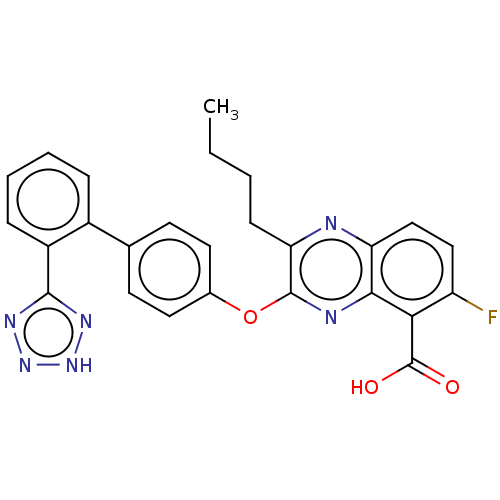 (CHEMBL74445) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231142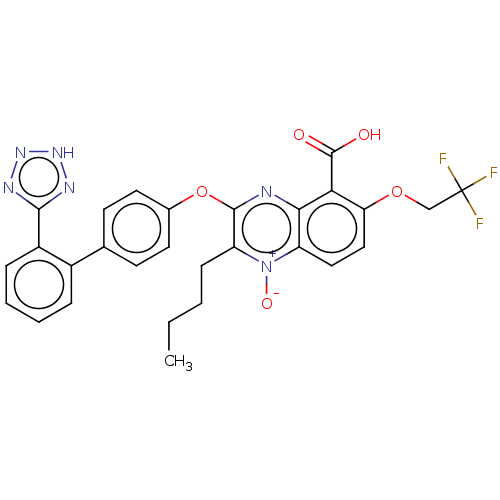 (CHEMBL312068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231148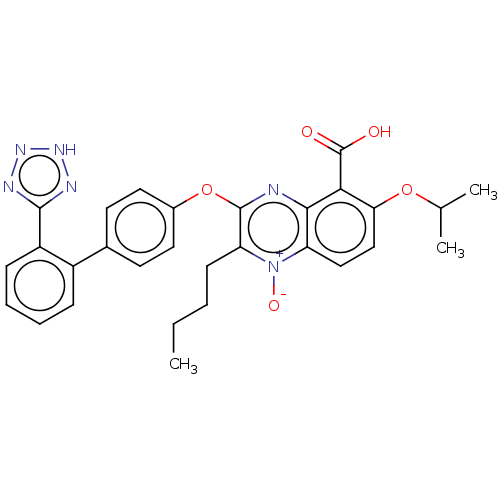 (CHEMBL77376) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231011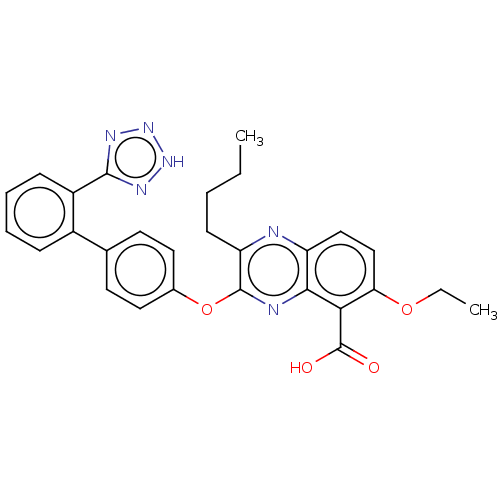 (CHEMBL309489) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231145 (CHEMBL76785) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231009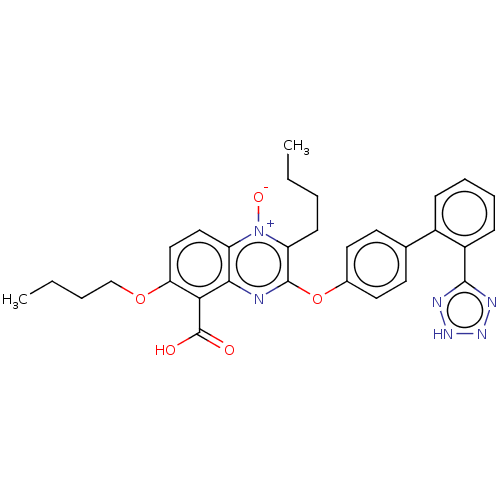 (CHEMBL76765) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231147 (CHEMBL433310) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231008 (CHEMBL75101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231008 (CHEMBL75101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231144 (CHEMBL76943) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231141 (CHEMBL77266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231146 (CHEMBL77185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231146 (CHEMBL77185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231008 (CHEMBL75101) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.22% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231144 (CHEMBL76943) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.22% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231006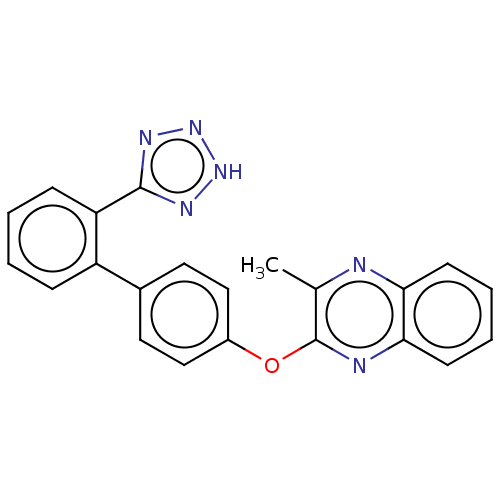 (CHEMBL307946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231006 (CHEMBL307946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231146 (CHEMBL77185) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.22% against rat adrenal Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50231006 (CHEMBL307946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.22% against rat Angiotensin II receptor, type 1 | J Med Chem 36: 2335-42 (1993) Article DOI: 10.1021/jm00068a010 BindingDB Entry DOI: 10.7270/Q21V5H6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||