 Found 67 hits Enz. Inhib. hit(s) with all data for entry = 50034312
Found 67 hits Enz. Inhib. hit(s) with all data for entry = 50034312 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50242333

((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...)Show SMILES CSCC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](S)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C39H66N10O9S2/c1-22(2)20-29(38(57)58)48-35(54)28(16-19-60-5)45-34(53)26(14-9-10-17-40)47-37(56)31(24(4)50)49-32(51)23(3)44-33(52)27(15-11-18-43-39(41)42)46-36(55)30(59)21-25-12-7-6-8-13-25/h6-8,12-13,22-24,26-31,50,59H,9-11,14-21,40H2,1-5H3,(H,44,52)(H,45,53)(H,46,55)(H,47,56)(H,48,54)(H,49,51)(H,57,58)(H4,41,42,43)/t23-,24+,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum botulinum neurotoxin type A light chain |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360392
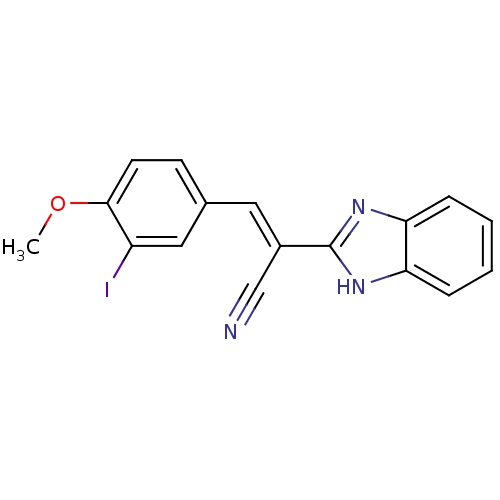
(CHEMBL1933865)Show InChI InChI=1S/C17H12IN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360392

(CHEMBL1933865)Show InChI InChI=1S/C17H12IN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain after 40 mins by HPLC assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360393
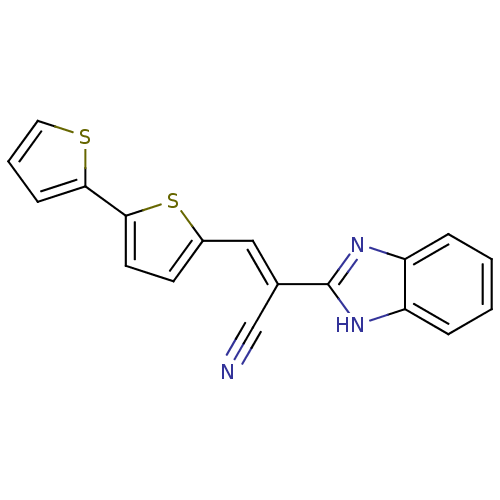
(CHEMBL1933882)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3S2/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage preincubated for 90 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360393

(CHEMBL1933882)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3S2/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360393

(CHEMBL1933882)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3S2/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain after 40 mins by HPLC assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360387
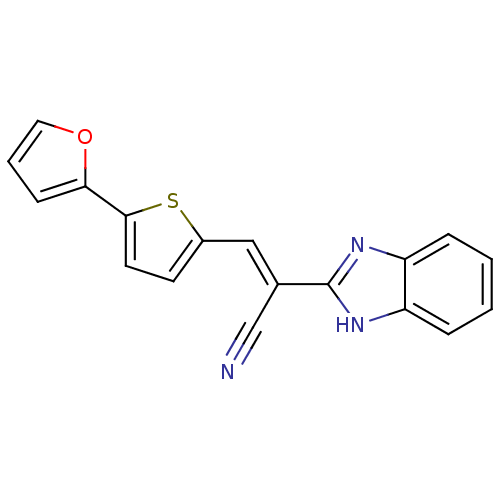
(CHEMBL1933884)Show SMILES N#C\C(=C/c1ccc(s1)-c1ccco1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3OS/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360400
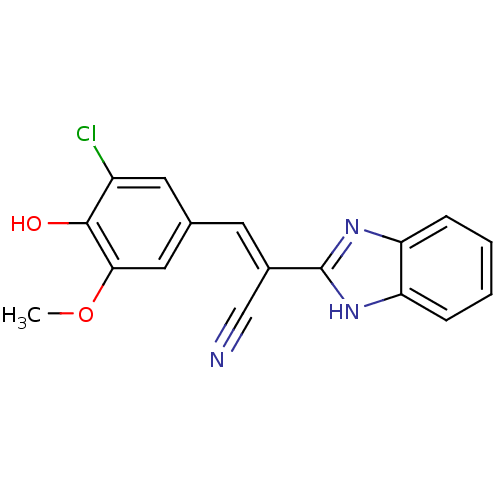
(CHEMBL1933871)Show SMILES COc1cc(\C=C(/C#N)c2nc3ccccc3[nH]2)cc(Cl)c1O Show InChI InChI=1S/C17H12ClN3O2/c1-23-15-8-10(7-12(18)16(15)22)6-11(9-19)17-20-13-4-2-3-5-14(13)21-17/h2-8,22H,1H3,(H,20,21)/b11-6+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360403
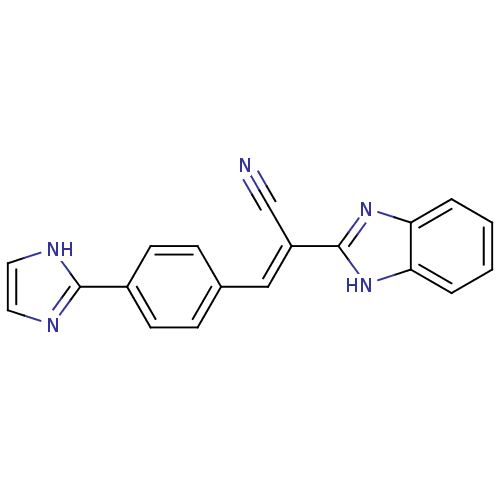
(CHEMBL1933874)Show SMILES N#C\C(=C/c1ccc(cc1)-c1ncc[nH]1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C19H13N5/c20-12-15(19-23-16-3-1-2-4-17(16)24-19)11-13-5-7-14(8-6-13)18-21-9-10-22-18/h1-11H,(H,21,22)(H,23,24)/b15-11+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50360392

(CHEMBL1933865)Show InChI InChI=1S/C17H12IN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360402
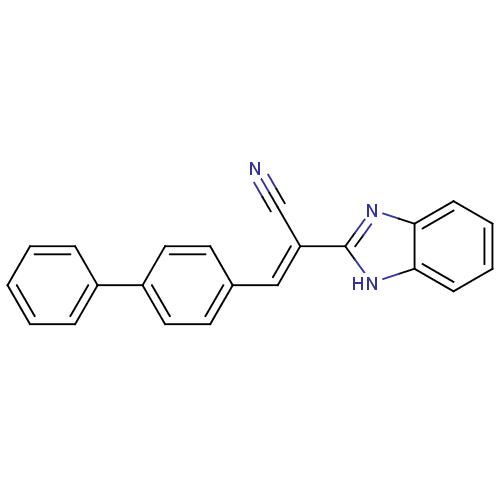
(CHEMBL1933873)Show SMILES N#C\C(=C/c1ccc(cc1)-c1ccccc1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H15N3/c23-15-19(22-24-20-8-4-5-9-21(20)25-22)14-16-10-12-18(13-11-16)17-6-2-1-3-7-17/h1-14H,(H,24,25)/b19-14+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360401
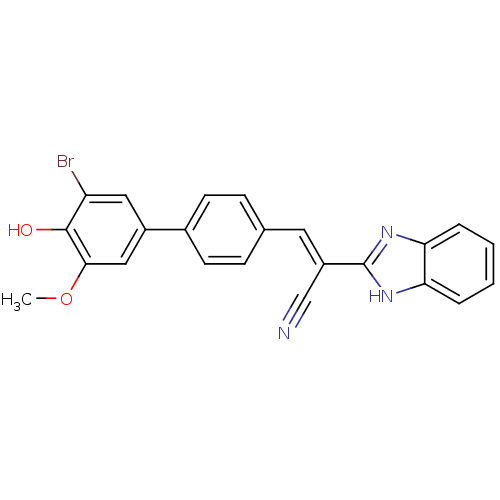
(CHEMBL1933872)Show SMILES COc1cc(cc(Br)c1O)-c1ccc(\C=C(/C#N)c2nc3ccccc3[nH]2)cc1 Show InChI InChI=1S/C23H16BrN3O2/c1-29-21-12-16(11-18(24)22(21)28)15-8-6-14(7-9-15)10-17(13-25)23-26-19-4-2-3-5-20(19)27-23/h2-12,28H,1H3,(H,26,27)/b17-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50360393

(CHEMBL1933882)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3S2/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50360392

(CHEMBL1933865)Show InChI InChI=1S/C17H12IN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP9 |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM49541
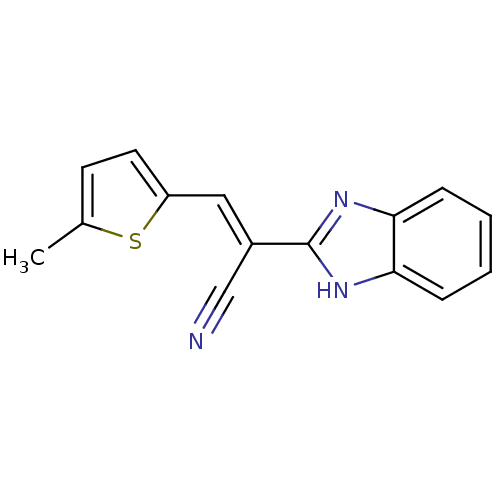
((E)-2-(1H-benzimidazol-2-yl)-3-(5-methyl-2-thienyl...)Show InChI InChI=1S/C15H11N3S/c1-10-6-7-12(19-10)8-11(9-16)15-17-13-4-2-3-5-14(13)18-15/h2-8H,1H3,(H,17,18)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50360386
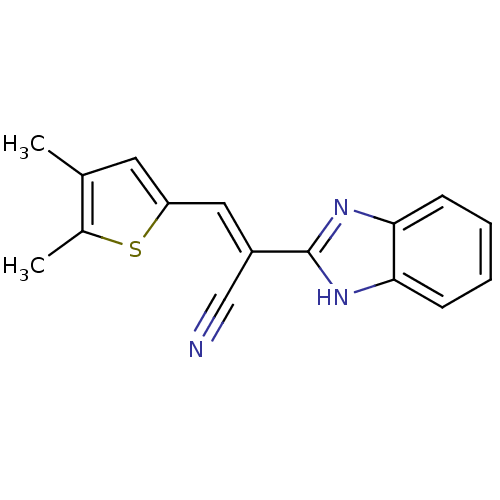
(CHEMBL1933883)Show InChI InChI=1S/C16H13N3S/c1-10-7-13(20-11(10)2)8-12(9-17)16-18-14-5-3-4-6-15(14)19-16/h3-8H,1-2H3,(H,18,19)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM42055
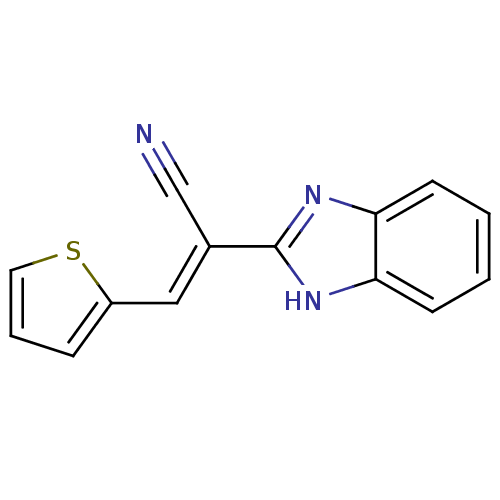
((E)-2-(1H-benzimidazol-2-yl)-3-(2-thienyl)acryloni...)Show InChI InChI=1S/C14H9N3S/c15-9-10(8-11-4-3-7-18-11)14-16-12-5-1-2-6-13(12)17-14/h1-8H,(H,16,17)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50360387

(CHEMBL1933884)Show SMILES N#C\C(=C/c1ccc(s1)-c1ccco1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3OS/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50360388
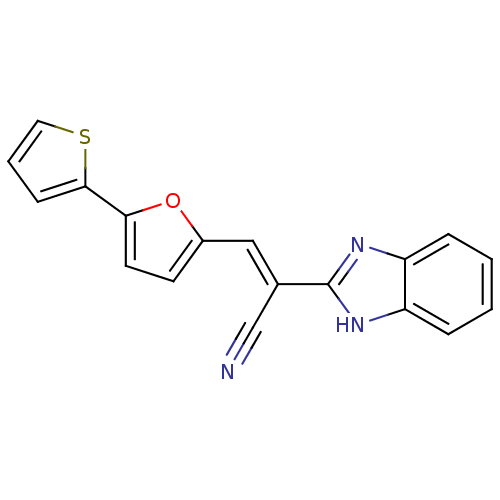
(CHEMBL1933885)Show SMILES N#C\C(=C/c1ccc(o1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3OS/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-16(22-13)17-6-3-9-23-17/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50360389
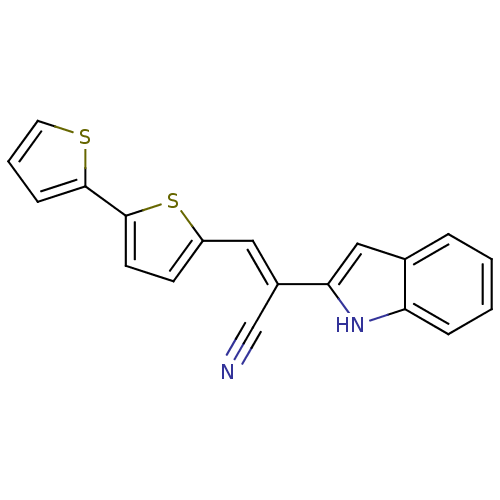
(CHEMBL1933886)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C19H12N2S2/c20-12-14(17-11-13-4-1-2-5-16(13)21-17)10-15-7-8-19(23-15)18-6-3-9-22-18/h1-11,21H/b14-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50360390
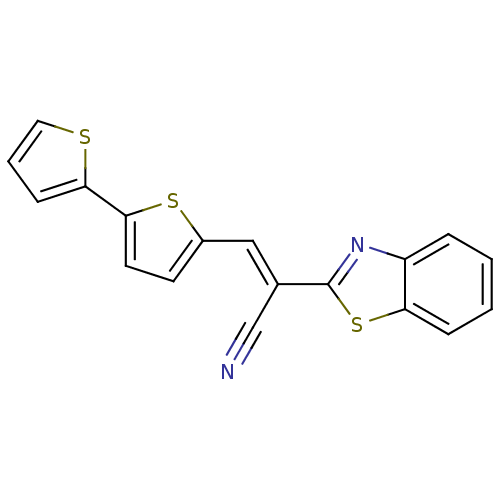
(CHEMBL1933887)Show InChI InChI=1S/C18H10N2S3/c19-11-12(18-20-14-4-1-2-5-15(14)23-18)10-13-7-8-17(22-13)16-6-3-9-21-16/h1-10H/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Lethal factor
(Bacillus anthracis) | BDBM50360391
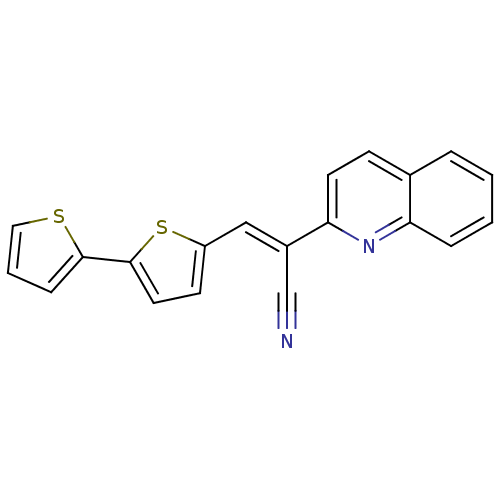
(CHEMBL1933889)Show InChI InChI=1S/C20H12N2S2/c21-13-15(18-9-7-14-4-1-2-5-17(14)22-18)12-16-8-10-20(24-16)19-6-3-11-23-19/h1-12H/b15-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Bacillus anthracis lethal factor using MCA-KKVYPYPME[dnp]Kamide peptide substrate after 30 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type B
(Clostridium botulinum) | BDBM50360392

(CHEMBL1933865)Show InChI InChI=1S/C17H12IN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/B light chain after 40 mins by FRET-based assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type B
(Clostridium botulinum) | BDBM50360393

(CHEMBL1933882)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3S2/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/B light chain after 40 mins by FRET-based assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50360392

(CHEMBL1933865)Show InChI InChI=1S/C17H12IN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP1 |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50360393

(CHEMBL1933882)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3S2/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP1 |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50360392

(CHEMBL1933865)Show InChI InChI=1S/C17H12IN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50360393

(CHEMBL1933882)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3S2/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360394
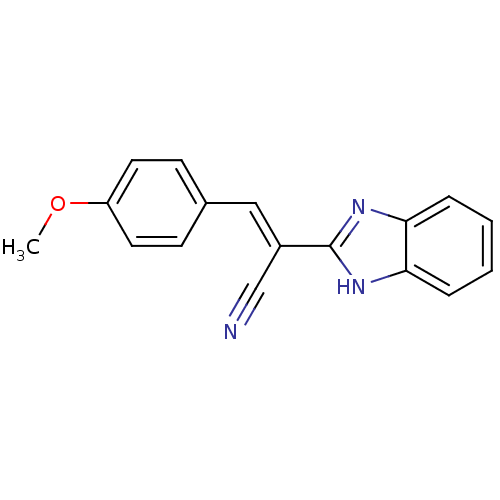
(CHEMBL116847)Show InChI InChI=1S/C17H13N3O/c1-21-14-8-6-12(7-9-14)10-13(11-18)17-19-15-4-2-3-5-16(15)20-17/h2-10H,1H3,(H,19,20)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360395
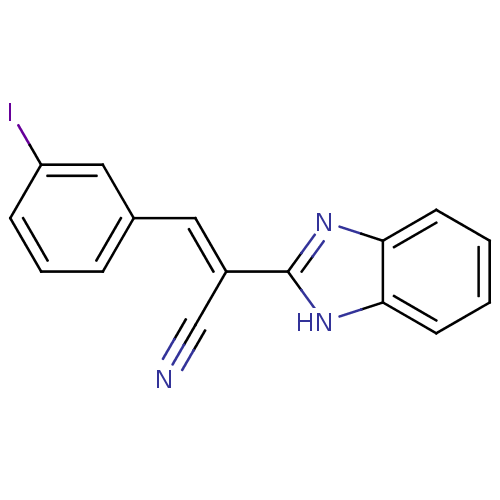
(CHEMBL1933866)Show InChI InChI=1S/C16H10IN3/c17-13-5-3-4-11(9-13)8-12(10-18)16-19-14-6-1-2-7-15(14)20-16/h1-9H,(H,19,20)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360396
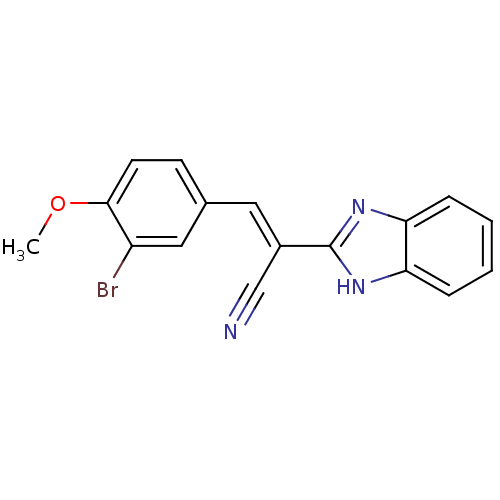
(CHEMBL1933867)Show InChI InChI=1S/C17H12BrN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360397

(CHEMBL1933868)Show InChI InChI=1S/C17H12ClN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360398
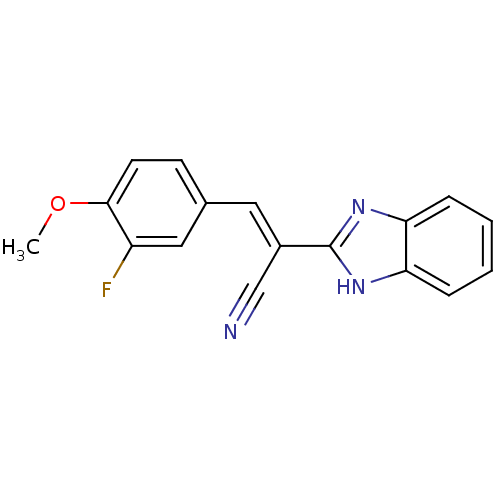
(CHEMBL1933869)Show InChI InChI=1S/C17H12FN3O/c1-22-16-7-6-11(9-13(16)18)8-12(10-19)17-20-14-4-2-3-5-15(14)21-17/h2-9H,1H3,(H,20,21)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
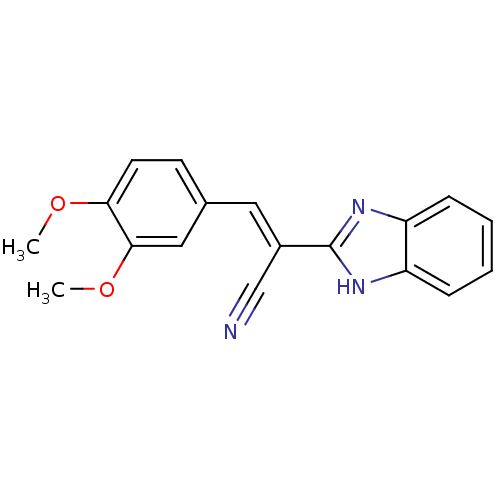
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360399
(CHEMBL1933870)Show InChI InChI=1S/C18H15N3O2/c1-22-16-8-7-12(10-17(16)23-2)9-13(11-19)18-20-14-5-3-4-6-15(14)21-18/h3-10H,1-2H3,(H,20,21)/b13-9+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
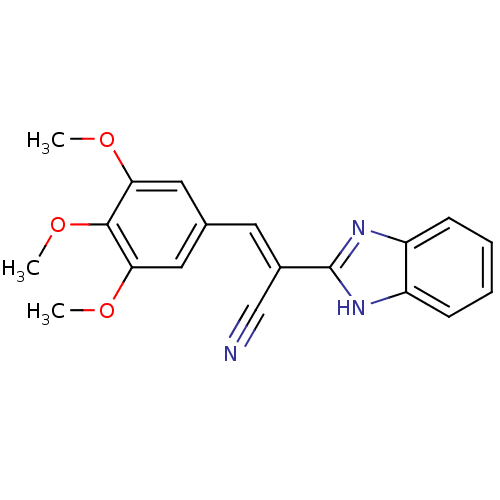
(Clostridium botulinum) | BDBM50360404
(CHEMBL1933875)Show SMILES COc1cc(\C=C(/C#N)c2nc3ccccc3[nH]2)cc(OC)c1OC Show InChI InChI=1S/C19H17N3O3/c1-23-16-9-12(10-17(24-2)18(16)25-3)8-13(11-20)19-21-14-6-4-5-7-15(14)22-19/h4-10H,1-3H3,(H,21,22)/b13-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360405
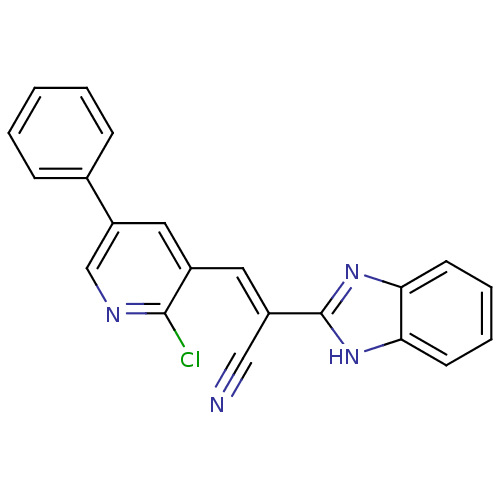
(CHEMBL1933876)Show SMILES Clc1ncc(cc1\C=C(/C#N)c1nc2ccccc2[nH]1)-c1ccccc1 Show InChI InChI=1S/C21H13ClN4/c22-20-15(11-17(13-24-20)14-6-2-1-3-7-14)10-16(12-23)21-25-18-8-4-5-9-19(18)26-21/h1-11,13H,(H,25,26)/b16-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360406
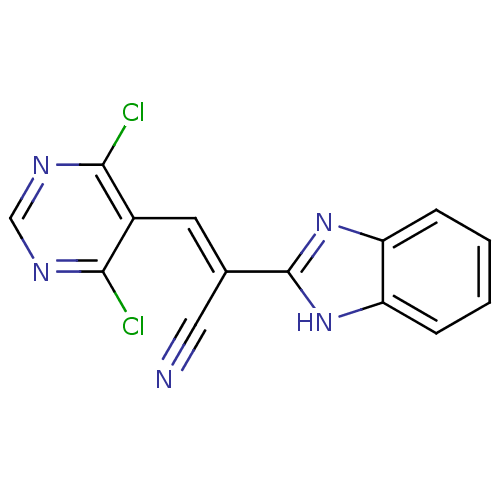
(CHEMBL1933877)Show InChI InChI=1S/C14H7Cl2N5/c15-12-9(13(16)19-7-18-12)5-8(6-17)14-20-10-3-1-2-4-11(10)21-14/h1-5,7H,(H,20,21)/b8-5+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360407
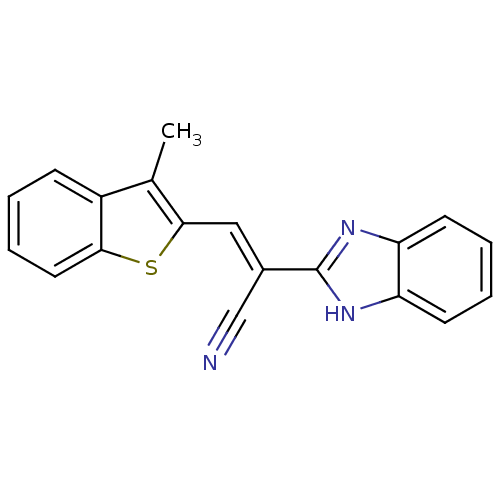
(CHEMBL1933878)Show InChI InChI=1S/C19H13N3S/c1-12-14-6-2-5-9-17(14)23-18(12)10-13(11-20)19-21-15-7-3-4-8-16(15)22-19/h2-10H,1H3,(H,21,22)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360408
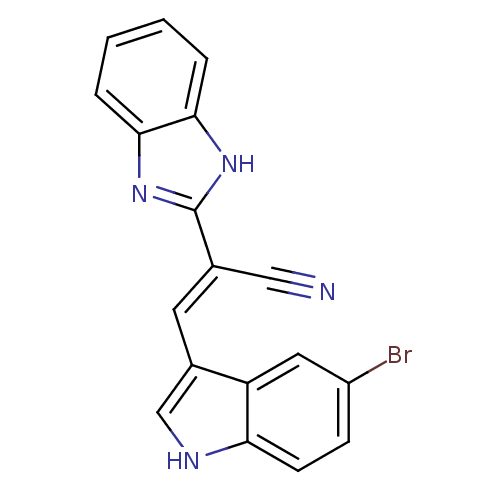
(CHEMBL1933879)Show SMILES Brc1ccc2[nH]cc(\C=C(/C#N)c3nc4ccccc4[nH]3)c2c1 Show InChI InChI=1S/C18H11BrN4/c19-13-5-6-15-14(8-13)12(10-21-15)7-11(9-20)18-22-16-3-1-2-4-17(16)23-18/h1-8,10,21H,(H,22,23)/b11-7+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360409
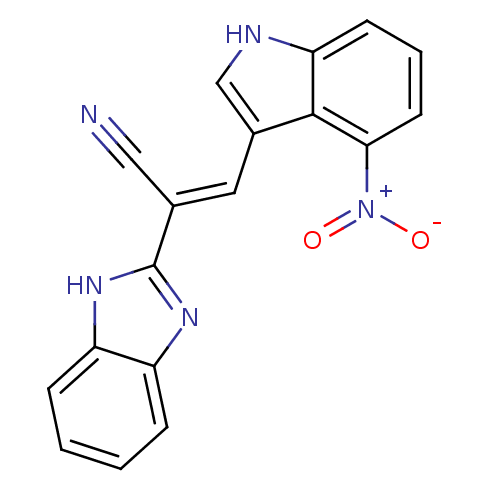
(CHEMBL1933880)Show SMILES [O-][N+](=O)c1cccc2[nH]cc(\C=C(/C#N)c3nc4ccccc4[nH]3)c12 Show InChI InChI=1S/C18H11N5O2/c19-9-11(18-21-13-4-1-2-5-14(13)22-18)8-12-10-20-15-6-3-7-16(17(12)15)23(24)25/h1-8,10,20H,(H,21,22)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360410
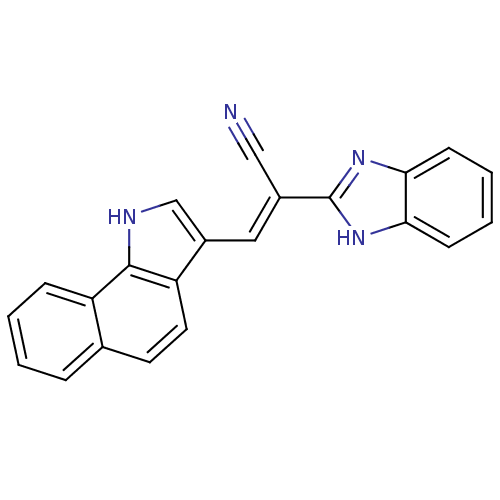
(CHEMBL1933881)Show SMILES N#C\C(=C/c1c[nH]c2c1ccc1ccccc21)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H14N4/c23-12-15(22-25-19-7-3-4-8-20(19)26-22)11-16-13-24-21-17-6-2-1-5-14(17)9-10-18(16)21/h1-11,13,24H,(H,25,26)/b15-11+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM49541

((E)-2-(1H-benzimidazol-2-yl)-3-(5-methyl-2-thienyl...)Show InChI InChI=1S/C15H11N3S/c1-10-6-7-12(19-10)8-11(9-16)15-17-13-4-2-3-5-14(13)18-15/h2-8H,1H3,(H,17,18)/b11-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360386

(CHEMBL1933883)Show InChI InChI=1S/C16H13N3S/c1-10-7-13(20-11(10)2)8-12(9-17)16-18-14-5-3-4-6-15(14)19-16/h3-8H,1-2H3,(H,18,19)/b12-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM42055

((E)-2-(1H-benzimidazol-2-yl)-3-(2-thienyl)acryloni...)Show InChI InChI=1S/C14H9N3S/c15-9-10(8-11-4-3-7-18-11)14-16-12-5-1-2-6-13(12)17-14/h1-8H,(H,16,17)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360388

(CHEMBL1933885)Show SMILES N#C\C(=C/c1ccc(o1)-c1cccs1)c1nc2ccccc2[nH]1 Show InChI InChI=1S/C18H11N3OS/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-16(22-13)17-6-3-9-23-17/h1-10H,(H,20,21)/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360389

(CHEMBL1933886)Show SMILES N#C\C(=C/c1ccc(s1)-c1cccs1)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C19H12N2S2/c20-12-14(17-11-13-4-1-2-5-16(13)21-17)10-15-7-8-19(23-15)18-6-3-9-22-18/h1-11,21H/b14-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360390

(CHEMBL1933887)Show InChI InChI=1S/C18H10N2S3/c19-11-12(18-20-14-4-1-2-5-15(14)23-18)10-13-7-8-17(22-13)16-6-3-9-21-16/h1-10H/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360411
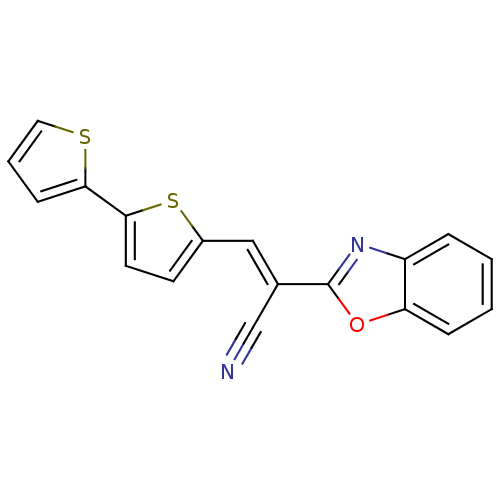
(CHEMBL1933888)Show InChI InChI=1S/C18H10N2OS2/c19-11-12(18-20-14-4-1-2-5-15(14)21-18)10-13-7-8-17(23-13)16-6-3-9-22-16/h1-10H/b12-10+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360391

(CHEMBL1933889)Show InChI InChI=1S/C20H12N2S2/c21-13-15(18-9-7-14-4-1-2-5-17(14)22-18)12-16-8-10-20(24-16)19-6-3-11-23-19/h1-12H/b15-12+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50360412
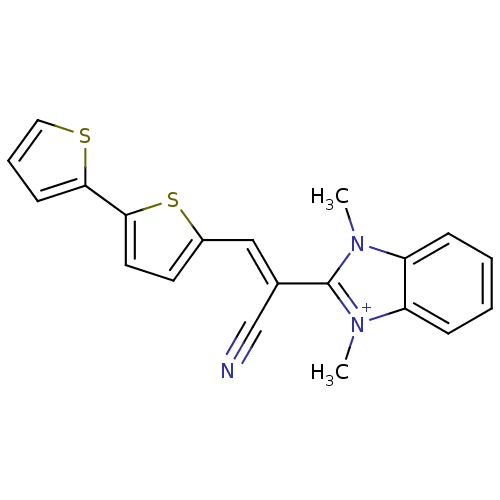
(CHEMBL1933890)Show SMILES Cn1c(\C(=C\c2ccc(s2)-c2cccs2)C#N)[n+](C)c2ccccc12 Show InChI InChI=1S/C20H16N3S2/c1-22-16-6-3-4-7-17(16)23(2)20(22)14(13-21)12-15-9-10-19(25-15)18-8-5-11-24-18/h3-12H,1-2H3/q+1/b14-12+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Microbiotix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain-mediated SNAP-25 substrate cleavage after 40 mins by FRET assay |
Bioorg Med Chem 19: 7338-48 (2011)
Article DOI: 10.1016/j.bmc.2011.10.062
BindingDB Entry DOI: 10.7270/Q2FF3SS3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data